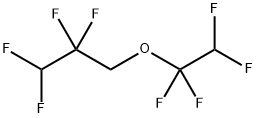
1,1,2,2-Tetrafluoroethyl-2,2,3,3-tetrafluoropropylether synthesis
- Product Name:1,1,2,2-Tetrafluoroethyl-2,2,3,3-tetrafluoropropylether
- CAS Number:16627-68-2
- Molecular formula:C5H4F8O
- Molecular Weight:232.07
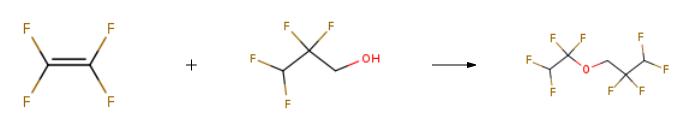
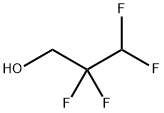
A system of a 6-L autoclave made of stainless steel was evacuated. The autoclave was charged with 401 g of potassium hydroxide (7.15 mol, 0.55 molar amount relative to 1 mol of a fluorine-containing alkyl alcohol), water (1604 mL), 2,2,3,3-tetrafluoro-1-propanol (boiling point of 109° C., specific gravity of 1.4) represented by HCF2CF2CH2OH (1716 g, 13 mol) as a fluorine-containing alkyl alcohol. Then, the system was evacuated and replaced with nitrogen 20 times at room temperature. After evacuated, the system was filled with tetrafluoroethylene to 0.1 MPa, and the reaction system was heated to 85° C. After the temperature in the system reached 85° C., tetrafluoroethylene was added thereto little by little so that the reaction pressure was kept at 0.5 to 0.8 MPa. The temperature in the system was adjusted so as to be kept at 75 to 95° C.Tetrafluoroethylene was added until the amount reached 0.5 molar relative to 1 mol of the fluorine-containing alkyl alcohol. The reaction was continuously carried out under stirring. When reduction in the pressure in the autoclave was stopped, the temperature in the autoclave was reduced to room temperature and unreacted tetrafluoroethylene was discharged, and the reaction was stopped. These steps took 5 hours to perform.The fluoroether of a lower phase of the production solution was HCF2CF2CH2OCF2CF2H (boiling point of 92° C., specific gravity of 1.52). Table 1 shows the composition of the fluoroether production solution of a lower phase resulting from GC analysis.
Yield:16627-68-2 98.5%
Reaction Conditions:
with potassium hydroxide in water at 75 - 95; under 5625.56 - 6000.6 Torr; for 8.5 h;Product distribution / selectivity;Inert atmosphere;Autoclave;
Steps:
8
EXAMPLE 1 Step (A) The inside of a 6-liter stainless steel autoclave was evacuated, and after introducing potassium hydroxide (546 g: 9.75 mole), water (2,184 ml) and 2,2,3,3-tetrafluoropropyl alcohol (fluorine-containing alkyl alcohol): HCF2CF2CH2OH (1,716 g: 13 mole), evacuation and replacement with nitrogen gas of the inside of the autoclave were conducted 20 times at room temperature. After evacuation of the system, tetrafluoroethylene was introduced to give the inside pressure of the system of 0.1 MPa, and heating was conducted to give the inside temperature of 75°C. After the inside temperature had reached 75°C, tetrafluoroethylene was added little by little so as to maintain the reaction pressure at 0.7 MPa to 0.8 MPa. The inside temperature was adjusted so as to be maintained at 75°C to 95°C.Step (B) When the amount of added tetrafluoroethylene reached 0.7 mole to 1 mole of fluorine-containing alkyl alcohol, supply of tetrafluoroethylene was stopped and the reaction was continued with stirring. When lowering of the inside pressure of the autoclave stopped, the inside temperature of the autoclave was brought to room temperature and unreacted tetrafluoroethylene was discharged to terminate the reaction. In the autoclave, a solution of reaction product separated into two layers of a lower organic layer (ether layer, specific gravity: 1.6) and an upper water layer had been formed. After sampling the upper water layer, the amounts of potassium fluoride and potassium difluoroacetate which were products by the side reaction of tetrafluoroethylene and potassium hydroxide and further the amount of unreacted fluorine-containing alkyl alcohol were determined by 19F-NMR. Potassium fluoride was 3.0 mole, potassium difluoroacetate was 1.4 mole, and unreacted fluorine-containing alkyl alcohol was 7.1 mole.Step (D) From the amounts of potassium fluoride and potassium difluoroacetate determined above, the amount of consumed potassium hydroxide was calculated (3.0 mole + 1.4 mole = 4.4 mole), and a consumed amount (246 g: 4.4 mole) of potassium hydroxide was added to the water layer remaining in the solution of reaction product.Step (C) Then, after washing the lower organic layer in the solution of reaction product with water once, the organic layer was separated and recovered. The amount of recovered organic layer was 1,369 g, and as a result of 19F-NMR and 1H-NMR analyses, the organic layer contained fluorine-containing ether represented by HCF2CF2CH2OCF2CF2H and its purity measured by GC was 99.72 %. The amount of this produced fluorine-containing ether (1,369 g × 0.9972 = 1,365 g: 5.9 mole) was an amount corresponding to 47.4 % when converted to the conversion ratio of the fluorine-containing alkyl alcohol. At this stage, the yield was 42.3 %.Step (E) From the amount of unreacted fluorine-containing alkyl alcohol determined above, the amount of consumed fluorine-containing alkyl alcohol was calculated (Charged fluorine-containing alkyl alcohol (13 mole) - unreacted fluorine-containing alkyl alcohol (7.1 mole) = 5.9 mole), and a consumed amount (779 g: 5.9 mole) of fluorine-containing alkyl alcohol was added to the water layer remaining in the solution of reaction product.Step (F) 1,357 g of the organic layer obtained in the step (C) was subjected to simple distillation, and fluorine-containing ether having purity (GC analysis) of 99.73 % was obtained at yield of 95 %.Repeating the steps after the step (A) After completion of the step (E), the procedures of the step (A) were repeated and then the reaction was stopped under the same condition (conversion ratio: 45 %) as in the step (B) and the produced organic layer was separated and recovered (step (C)). The fluorine-containing ether was recovered in an amount of 1,290 g at purity (GC analysis) of 99.76 %, and further was subjected to simple distillation (step (F)). As a result, fluorine-containing ether having purity (GC analysis) of 99.77 % was obtained at yield of 97.0 %.; EXAMPLE 8 The steps (A) to (F) were carried out in the same manner as in Example 1 except that in the step (A), the reaction pressure was changed as shown in Table 1, and the amount of potassium hydroxide was changed to 400 g (7.15 mole) which was 0.55 mole to 1 mole of the fluorine-containing alkyl alcohol. The results are shown in Table 1.
References:
EP2305626,2011,A1 Location in patent:Page/Page column 6-8; 11