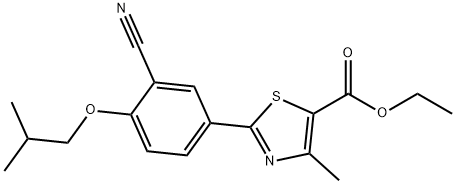
Ethyl 2-(3-cyano-4-isobutoxyphenyl)-4-methyl-5-thiazolecarboxylate synthesis
- Product Name:Ethyl 2-(3-cyano-4-isobutoxyphenyl)-4-methyl-5-thiazolecarboxylate
- CAS Number:160844-75-7
- Molecular formula:C18H20N2O3S
- Molecular Weight:344.43
78-77-3
338 suppliers
$14.00/5g
161798-01-2
375 suppliers
$6.00/250mg

160844-75-7
393 suppliers
$11.00/250mg
Yield:160844-75-7 84%
Reaction Conditions:
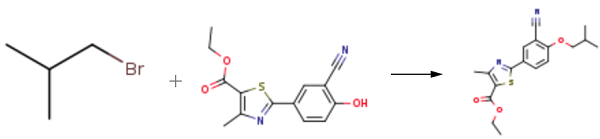
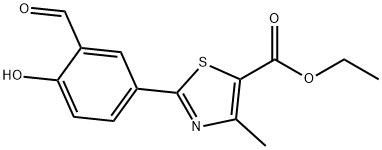
Stage #1:ethyl 2‐(3‐formyl‐4‐hydroxyphenyl)‐4‐methylthiazole‐5‐carboxylate with hydroxylamine hydrochloride in dimethyl sulfoxide at 40; for 0.5 h;
Stage #2: with acetyl chloride in dimethyl sulfoxide at 70 - 80;
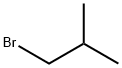
Stage #3:Isobutyl bromide with potassium carbonate in dimethyl sulfoxide at 20 - 80;
Steps:
1
Example - 1: Preparation of Ethyl-2-(3-cyano-4-isobutoxy phenyl)-4-methyI thiozole -5-carboxylateA mixture of 10. Og of Ethyl -2-(3-formyl-4-hydroxy phenyl)-4-methyl thiozole -5- carboxylate and 2.85 g of hydroxylamine hydrochloride were stirred for 30 minutes in 40 g of Dimethyl sulfoxide. To this reaction mixture 3.3 grams of acetyl chloride was added and stirred at 70 -80°C for 2-3 hours. Reaction mass was cooled to room temperature and to this 19 g of potassium carbonate and 19 g of isobutyl bromide was added successively. The reaction mass was stirred for 5 hours at 70-80°C. Reaction mass was diluted with 200 ml of purified water. The reaction mass was filtered and washed with purified water to give 10.0 g of Ethyl-2-(3-cyano-4-isobutoxy phenyl)-4-methyl thiozole -5-carboxyltae (yield 84.0%)
References:
MATRIX LABORATORIES LTD;VELLENKI, Siva Rama Prasad;ARABINDA, Sahu;RAAVI, Satyanarayana;NUCHU, Ravi;DANDALA, Ramesh WO2012/66561, 2012, A1 Location in patent:Page/Page column 8

78-77-3
338 suppliers
$14.00/5g
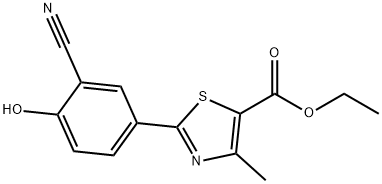
161798-02-3
228 suppliers
inquiry

160844-75-7
393 suppliers
$11.00/250mg
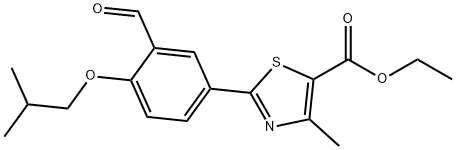
161798-03-4
330 suppliers
$18.00/100mg

160844-75-7
393 suppliers
$11.00/250mg
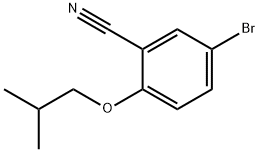
876918-26-2
12 suppliers
inquiry

20582-55-2
195 suppliers
$9.00/1g

160844-75-7
393 suppliers
$11.00/250mg
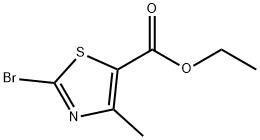
22900-83-0
186 suppliers
$5.00/250mg
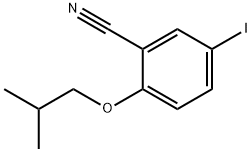
1139901-87-3
5 suppliers
inquiry

160844-75-7
393 suppliers
$11.00/250mg