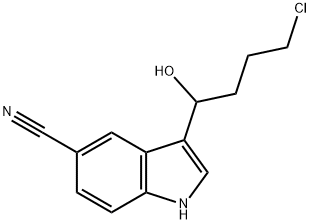
3-(4-Chloro-1-hydroxybutyl)-1H-indole-5-carbonitrile synthesis
- Product Name:3-(4-Chloro-1-hydroxybutyl)-1H-indole-5-carbonitrile
- CAS Number:1451194-34-5
- Molecular formula:C13H13ClN2O
- Molecular Weight:248.71
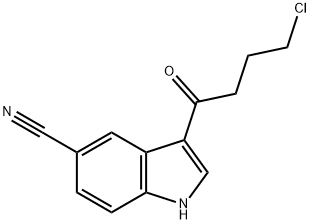
276863-95-7
87 suppliers
inquiry

1451194-34-5
14 suppliers
inquiry
Yield:1451194-34-5 73.5%
Reaction Conditions:
with sodium tetrahydroborate;water;sodium hydroxide in tetrahydrofuran at 30 - 35; for 3 h;
Steps:
2 Synthesis of the Intermediate Compound 3-(4-Chloro-1-hydroxybutyl)-1H-indol-5-carbonitrile
3-(4-Chlorobutyryl)-1H-indol-5-carbonitrile58 gTetrahydrofuran116 gDistilled water11.6 gHeat at 30-35° C., then add a separately prepared solution of:Distilled water29 g30% NaOH0.3 gSodium borohydride7 gStir at 35° C. for 3 hours then addMethylene chloride116 gStir at 35° C., separate the lower organic phase then dry then addMethanol72.5 gDistilled water29 gStir at 35° C. to crystallization, cool at 5° C., filter and wash withDistilled water11.6 gDryThere are obtained 43 g; Yield: 73.5%
References:
US2013/225818,2013,A1 Location in patent:Paragraph 0077; 0078
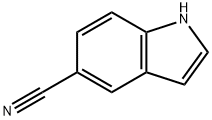
15861-24-2
449 suppliers
$10.00/5g

1451194-34-5
14 suppliers
inquiry