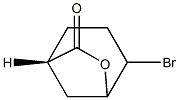
(1S,4S,5S)-4-Bromo-6-oxabicyclo[3.2.1]octan-7-one synthesis
- Product Name:(1S,4S,5S)-4-Bromo-6-oxabicyclo[3.2.1]octan-7-one
- CAS Number:139893-81-5
- Molecular formula:C7H9BrO2
- Molecular Weight:205.05
5708-19-0
281 suppliers
$6.00/1g
![(1S,4S,5S)-4-Bromo-6-oxabicyclo[3.2.1]octan-7-one](/CAS/20200515/GIF/139893-81-5.gif)
139893-81-5
25 suppliers
inquiry
Yield:139893-81-5 91%
Reaction Conditions:
with N-Bromosuccinimide;sodium hydrogencarbonate in dichloromethane at 5 - 25; for 2.33333 h;
Steps:
1 Example 1: Preparation of (1S,45,55)-4-bromo-6- oxabicyclo[3 .2.1 ]octan-7 -one
Example 1
Preparation of (1S,4S,5S)-4-bromo-6-oxabicyclo[3.2.1]octan-7-one
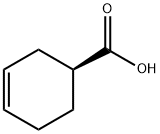
To a solution of (1 S)-3-cyclohexene-1-carboxylic acid (24.50 g, 194.2 mmol) in dichloromethane (125 mL) was charged sodium bicarbonate (17.13 g, 203.9 mmol), and the reaction mixture was cooled to 5-10° C. N-bromosuccinimide (36.29 g, 203.9 mmol) was added in four portions (over 20 minutes) while maintaining the temperature below 20° C.
The yellow to white suspension was warmed to room temperature and stirring continued for 2 hours until completion of the reaction as measured by TLC (thin-layer chromatography).
The reaction mixture was first treated with 10% aqueous sodium thiosulfate solution (122 mL) yielding a negative test for the presence of bromine using potassium iodide starch test paper, followed by slowly adding a solution of sodium phosphate monobasic (32.20 g, 233.4 mmol) in water (122 mL) due to effervescence.
After separation of the aqueous and organic phases, the organic phase was extracted with saturated ammonium chloride solution (2*122 mL) and the organic phase was dried over sodium sulfate and concentrated in vacuo to dryness to afford (1S,4S,5S)-4-bromo-6-oxabicyclo[3.2.1]octan-7-one as a white solid: 36.24 g (91% yield);
References:
US2018/179226,2018,A1 Location in patent:Paragraph 0247; 0248
67976-82-3
10 suppliers
inquiry
![(1S,4S,5S)-4-Bromo-6-oxabicyclo[3.2.1]octan-7-one](/CAS/20200515/GIF/139893-81-5.gif)
139893-81-5
25 suppliers
inquiry

5708-19-0
281 suppliers
$6.00/1g
3886-69-9
562 suppliers
$14.00/25G
![(1S,4S,5S)-4-Bromo-6-oxabicyclo[3.2.1]octan-7-one](/CAS/20200515/GIF/139893-81-5.gif)
139893-81-5
25 suppliers
inquiry
![6-Oxabicyclo[3.2.1]octan-7-one, 4-bromo-, (1R,4R,5R)-rel-](/CAS/20210305/GIF/19914-91-1.gif)
19914-91-1
0 suppliers
inquiry
![(1S,4S,5S)-4-Bromo-6-oxabicyclo[3.2.1]octan-7-one](/CAS/20200515/GIF/139893-81-5.gif)
139893-81-5
25 suppliers
inquiry