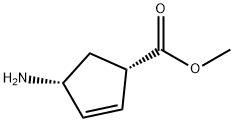
Methyl (1S,4R)-4-Amino-2-Cyclopentene-1-Carboxylate synthesis
- Product Name:Methyl (1S,4R)-4-Amino-2-Cyclopentene-1-Carboxylate
- CAS Number:138923-03-2
- Molecular formula:C7H11NO2
- Molecular Weight:141.17
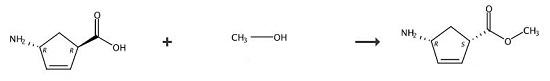
Weigh 50g of the by-product (+)1-amino-4-carboxy-2-cyclopentene extracted by γ-lactam enzymatic resolution and put it in a flask, add 200g of water, and slowly add dropwise at 25~30℃ Sulfuric acid, adjust the pH value of the solution to 1~2, after the dripping is completed, add 0.1g of the racemic catalyst o-phthalaldehyde, then heat to reflux, and react for 10~15h. Concentrate under reduced pressure to no fraction, add 200ml of methanol, 20g of tartaric acid, cool to 10-20°C, filter and wash with methanol. Then add 20g of thionyl chloride and 100ml of methanol to the filter cake, react at 65°C for 8h and concentrate under reduced pressure until there is no fraction. Add 100ml of dichloromethane, stir to dissolve and wash the dichloromethane layer twice with drinking water to obtain (- ) Methyl 1-amino-4-carboxylate-2-cyclopentene in dichloromethane solution, yield 42%.

67-56-1
746 suppliers
$9.00/25ml
![2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/49805-30-3.gif)
49805-30-3
365 suppliers
$5.00/5g

138923-03-2
32 suppliers
$333.00/250mg
Yield:138923-03-2 41.1%
Reaction Conditions:
Stage #1: methanol;2-azabicyclo[2.2.1.]hept-5-en-3-onewith thionyl chloride at 25;
Stage #2: with tartaric acid;triethylamine in water at 20;Temperature;
Steps:
1; 2; 3 Example 2
Dissolve 100 g of vins lactone (formula III) in 250 g of methanol, dropwise add 65.4 g of thionyl chloride, control the temperature at 25°C, and react for 2 hours after dropping. After the reaction is complete, distill methanol under reduced pressure, add 130g methanol to the concentrated product, mix well, add tartaric acid solution (60g water + 82.6g L-(+)-tartaric acid), control the temperature at 20°C, and drop in 61.20g triethyl ether amine. After dropping, keep stirring until solid precipitates, grow crystals for 6 hours, filter, and wash the filter cake with 50 g of methanol. The filter cake was dried and collected to obtain a white solid crude product. Put the crude product in 102g of water and 204g of methanol, heat up to 72°C and stir to dissolve, then cool down to 60°C, add 510g of methanol, a large amount of solid precipitates, cool down to -3°C for 1h, grow crystals for 3h, filter, dry the filter cake, and collect , to obtain 109.76 g of a white refined product, which is a compound having the structure shown in formula I, and the yield is 41.1%.
References:
CN115504908,2022,A Location in patent:Paragraph 0033; 0044-0045; 0048-0049; 0051-0052

67-56-1
746 suppliers
$9.00/25ml
![((1R,4S)-2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/79200-56-9.gif)
79200-56-9
221 suppliers
$10.00/1g

138923-03-2
32 suppliers
$333.00/250mg
![2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/49805-30-3.gif)
49805-30-3
365 suppliers
$5.00/5g

138923-03-2
32 suppliers
$333.00/250mg
![2-(hydroxyMethyl)-2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/157732-10-0.gif)
157732-10-0
1 suppliers
inquiry

138923-03-2
32 suppliers
$333.00/250mg
![2-Azabicyclo[2.2.1]hept-5-en-3-one, 2-(hydroxymethyl)-, (1R,4S)-](/CAS/20210305/GIF/157810-21-4.gif)
157810-21-4
0 suppliers
inquiry

138923-03-2
32 suppliers
$333.00/250mg