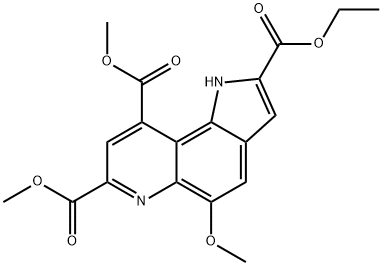
7,9-DIMETHOXYCARBONYL-2-ETHOXYCARBONYL-5-METHOXY-1H-PYRROLO-[2,3-F]QUINOLINE synthesis
- Product Name:7,9-DIMETHOXYCARBONYL-2-ETHOXYCARBONYL-5-METHOXY-1H-PYRROLO-[2,3-F]QUINOLINE
- CAS Number:133706-80-6
- Molecular formula:C19H18N2O7
- Molecular Weight:386.36
![1H-Pyrrolo[2,3-f]quinoline-2,7,9-tricarboxylic acid, 6,7,8,9-tetrahydro-9-hydroxy-5-methoxy-, 2-ethyl 7,9-dimethyl ester](/CAS/20210305/GIF/910885-13-1.gif)
910885-13-1
0 suppliers
inquiry
![7,9-DIMETHOXYCARBONYL-2-ETHOXYCARBONYL-5-METHOXY-1H-PYRROLO-[2,3-F]QUINOLINE](/StructureFile/ChemBookStructure22/GIF/CB1648887.gif)
133706-80-6
11 suppliers
inquiry
Yield:133706-80-6 86.1%
Reaction Conditions:
with hydrogenchloride;oxygen;copper acetylacetonate in dichloromethane at 20;
Steps:
1.G; h
Into a 3-L, 3-neck flask equipped with a mechanical stirrer, an addition funnel, a temperature probe, a heating mantle, and a condenser vented to a nitrogen line under static pressure were placed, ethyl 6-amino-5-methoxy-lH-indole-2-carboxylate (8) (213.6 g, 0.912 mol) and 1325 mL of methylene chloride. The green suspension was stirred under nitrogen and a solution of dimethyl 2-oxoglutaconate (12) (175.0 g, 1.02 mol) in 700 mL of methylene chloride was added at room temperature over a 10-min period. An additional 100 mL of EPO
References:
WO2006/102642,2006,A1 Location in patent:Page/Page column 9-10; 15-17; 1/6
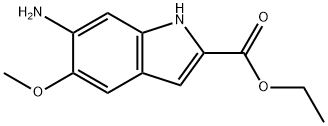
107575-60-0
73 suppliers
$95.09/1g
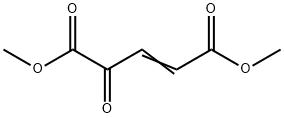
78939-37-4
28 suppliers
$130.00/250mg
![7,9-DIMETHOXYCARBONYL-2-ETHOXYCARBONYL-5-METHOXY-1H-PYRROLO-[2,3-F]QUINOLINE](/StructureFile/ChemBookStructure22/GIF/CB1648887.gif)
133706-80-6
11 suppliers
inquiry
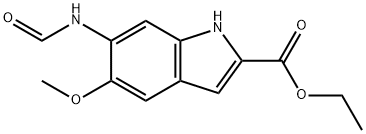
119825-27-3
13 suppliers
inquiry
![7,9-DIMETHOXYCARBONYL-2-ETHOXYCARBONYL-5-METHOXY-1H-PYRROLO-[2,3-F]QUINOLINE](/StructureFile/ChemBookStructure22/GIF/CB1648887.gif)
133706-80-6
11 suppliers
inquiry

107575-60-0
73 suppliers
$95.09/1g
![7,9-DIMETHOXYCARBONYL-2-ETHOXYCARBONYL-5-METHOXY-1H-PYRROLO-[2,3-F]QUINOLINE](/StructureFile/ChemBookStructure22/GIF/CB1648887.gif)
133706-80-6
11 suppliers
inquiry

78939-37-4
28 suppliers
$130.00/250mg
![7,9-DIMETHOXYCARBONYL-2-ETHOXYCARBONYL-5-METHOXY-1H-PYRROLO-[2,3-F]QUINOLINE](/StructureFile/ChemBookStructure22/GIF/CB1648887.gif)
133706-80-6
11 suppliers
inquiry