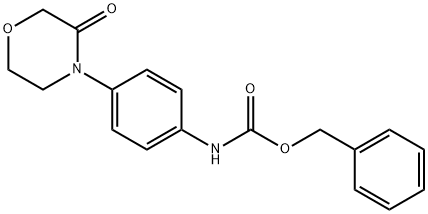
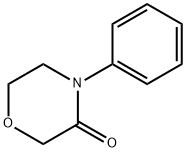
N-[4-(3-oxo-4-Morpholinyl)phenyl]carbaMic acid phenylMethyl ester synthesis
- Product Name:N-[4-(3-oxo-4-Morpholinyl)phenyl]carbaMic acid phenylMethyl ester
- CAS Number:1313613-18-1
- Molecular formula:C18H18N2O4
- Molecular Weight:326.35

501-53-1
395 suppliers
$10.00/1g

438056-69-0
609 suppliers
$10.00/5g
![N-[4-(3-oxo-4-Morpholinyl)phenyl]carbaMic acid phenylMethyl ester](/CAS/20150408/GIF/1313613-18-1.gif)
1313613-18-1
18 suppliers
inquiry
Yield:1313613-18-1 98%
Reaction Conditions:
with sodium hydrogencarbonate in water;acetone;toluene at 0 - 30; for 4 h;
Steps:
1 Example 1: Preparation of [4-(3-oxo-morpholin-4-yl)phenyl]carbamic acid benzyl ester
Example 1: Preparation of [4-(3-oxo-morpholin-4-yl)phenyl]carbamic acid benzyl ester (Compound-VIII, R = -OCH2Ph): 3.73g, (19.40 mmol ) of 4-(4-aminophenyl)morpholin-3-one and 80ml of acetone were charged in a clean and dry R.B. flask and stirred. A mixture of 40 ml of water and sodium bicarbonate (3.26g,38.80 mmol) was added. The resultant reaction mixture was cooled to about 0°C and benzyl chloroformate ( 50% w/w in toluene, 20.60 mmol) was added drop-wise. The reaction mixture was stirred at about 30°C for about 4 hours. After the completion of reaction, the reaction mixture was quenched by pouring into ice-water. The separated solid product was filtered and the solid was washed with water followed by n- hexane and dried to afford title compound as a white solid. Yield: 6.2 g, (% Yield: 98%).
References:
WO2013/105100,2013,A1 Location in patent:Page/Page column 49-50
348626-43-7
24 suppliers
inquiry
![N-[4-(3-oxo-4-Morpholinyl)phenyl]carbaMic acid phenylMethyl ester](/CAS/20150408/GIF/1313613-18-1.gif)
1313613-18-1
18 suppliers
inquiry
122-98-5
301 suppliers
$5.00/5G
![N-[4-(3-oxo-4-Morpholinyl)phenyl]carbaMic acid phenylMethyl ester](/CAS/20150408/GIF/1313613-18-1.gif)
1313613-18-1
18 suppliers
inquiry
446292-04-2
161 suppliers
inquiry
![N-[4-(3-oxo-4-Morpholinyl)phenyl]carbaMic acid phenylMethyl ester](/CAS/20150408/GIF/1313613-18-1.gif)
1313613-18-1
18 suppliers
inquiry
29518-11-4
105 suppliers
$17.00/250mg
![N-[4-(3-oxo-4-Morpholinyl)phenyl]carbaMic acid phenylMethyl ester](/CAS/20150408/GIF/1313613-18-1.gif)
1313613-18-1
18 suppliers
inquiry