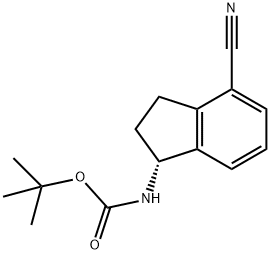
(R)-tert-butyl (4-cyano-2,3-dihydro-1H-inden-1-yl)carbamate synthesis
- Product Name:(R)-tert-butyl (4-cyano-2,3-dihydro-1H-inden-1-yl)carbamate
- CAS Number:1306763-30-3
- Molecular formula:C15H18N2O2
- Molecular Weight:258.32
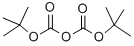
24424-99-5
833 suppliers
$13.50/25G
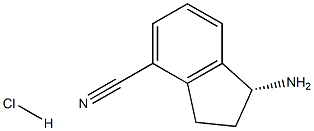
1306763-29-0
27 suppliers
inquiry

1306763-30-3
49 suppliers
inquiry
Yield: 84%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 2 h;
Steps:
52
[0458] (R )-tert-butyl 4-cyano-2, 3-dihydro-lH-inden-l-ylcarbamate (INT-52)[0459] To (R)-l-amino-2,3-dihydro-lH-indene-l-yl)-4-carbonitrile HC1 INT-50 (11.6 g, 59.6 mmol) in DCM (100 mL) at 0°C was added TEA (12.0 mL, 131.0 mmol). To the resulting solution was added a solution of Boc anhydride (14.3 g, 65.6 mmol) in DCM (30 mL) and the reaction mixture stirred at room temperature for 1.5 h. The reaction mixture was washed with brine, and the organic layers were dried over MgS04 and filtered. Additional DCM was added to a total volume of 250 mL and Norit (4.5 g) was added. The product was refluxed for 15 mins and the hot mixture filtered through a pad of celite / silica. The filtrate was concentrated and recrystallized from EA (50 mL) and hexane (150 mL) to produce 12.93 g (84%) of (R)-tert-butyl 4-cyano-2,3-dihydro-lH-inden-l-ylcarbamate INT-52 as an off-white solid. LCMS-ESI (m z) calculated for Ci5H18N202: 258.3; found 281.1 [M+Na]+, tR = 3.45 min. Elemental Analysis determined for Ci5H18N202; C calculated = 69.74%; found = 69.98%. H calculated = 7.02%; found = 7.14%. N calculated = 10.84%; found = 10.89%. NMR (400 MHz, CDC13) 6 7.64 - 7.49 (m, 2H), 7.34 (dt, J = 7.7, 3.8, 1H), 5.36 - 5.20 (m, 1H), 4.78 (d, J = 6.8, 1H), 3.20 (ddd, J = 16.9, 8.9, 3.3, lH), 3.02 (dt, J = 25.4, 8.4, 1H), 2.82 - 2.53 (m, 1H), 1.88 (dq, J = 13.2, 8.6, 1H), 1.55 - 1.44 (m, 9H). 13C NMR (101 MHz, DMSO) δ 155.52, 146.68, 146.32, 130.89, 128.70, 127.63, 117.51, 107.76, 77.98, 55.09, 31.88, 29.11, 28.19. Chiral HPLC: (R)-tert-butyl 4-cyano-2,3-dihydro-lH-inden-l- ylcarbamate was eluted using 2.5% EtOH in hexanes: >99.9% ee, tR = 19.36 min.
References:
RECEPTOS, INC.;MARTINBOROUGH, Esther;BOEHM, Marcus, F.;YEAGER, Adam, Richard;TAMIYA, Junko;HUANG, Liming;BRAHMACHARY, Enugurthi;MOORJANI, Manisha WO2011/60389, 2011, A1 Location in patent:Page/Page column 92-93

15115-60-3
329 suppliers
$5.00/1g

1306763-30-3
49 suppliers
inquiry
60899-34-5
204 suppliers
inquiry

1306763-30-3
49 suppliers
inquiry
1306763-27-8
2 suppliers
inquiry

1306763-30-3
49 suppliers
inquiry
1306763-28-9
5 suppliers
inquiry

1306763-30-3
49 suppliers
inquiry