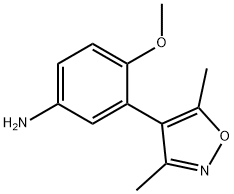
[3-(3,5-diMethyl-4-isoxazolyl)-4-(Methyloxy)phenyl]aMine synthesis
- Product Name:[3-(3,5-diMethyl-4-isoxazolyl)-4-(Methyloxy)phenyl]aMine
- CAS Number:1300031-61-1
- Molecular formula:C12H14N2O2
- Molecular Weight:218.25
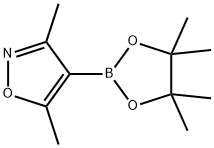
832114-00-8
229 suppliers
$6.00/250mg

74587-12-5
126 suppliers
$9.00/1g
![[3-(3,5-diMethyl-4-isoxazolyl)-4-(Methyloxy)phenyl]aMine](/CAS/20150408/GIF/1300031-61-1.gif)
1300031-61-1
1 suppliers
inquiry
Yield: 93%
Reaction Conditions:
with palladium diacetate;caesium carbonate;triphenylphosphine in 1,2-dimethoxyethane at 90; for 16 h;Inert atmosphere;
Steps:
1.5 Intermediate step 5 toward Example 1: 3-(3,5-dimethylisoxazol-4-yl)-4-methoxy-aniline
Intermediate step 5 toward Example 1: 3-(3,5-dimethylisoxazol-4-yl)-4-methoxy-aniline [00231] Pd(OAc)2 (1.42 g, 6.34 mmol) and PPh3 (3.33 g, 12.7 mmol) were weighed into a 2-necked flask equipped with a reflux condenser, and the flask was flushed with N2 gas. Degassed DME (60.0 mL) was added, and the mixture was stirred for 30 m at rt. 3,5-Dimethyl- 4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)isoxazole (34.0 g, 152 mmol), 3-iodo-4- methoxy-aniline (15.8 g, 63.4 mmol), and Cs2C03 (51.7 g, 159 mmol) were weighed into a second flask, and the flask was flushed with N2 gas. Degassed DME (300 mL) and degassed water (30.0 mL) were added. The mixture was stirred for 20 m and then added to the first flask. The second flask was rinsed with degassed DME (15.0 mL), and the liquid was transferred to the first flask. The resulting mixture was heated to 90°C for 16 h and then cooled to rt. The mixture was diluted with saturated aq NaHC03 (100 mL) and EtOAc (100 mL), and the aq phase was extracted with EtOAc (3X150 mL). The combined organic phases were dried over MgS04, filtered, and concentrated under reduced pressure. The product was purified by flash chromatography on silica gel, eluting with mixtures of EtOAc and hexanes to provide the title compound as a solid (12.9 g, 93%). 1H NMR (500 MHz, CDC13) δ 6.80 (d, J = 8.7 Hz, 1H), 6.69 (dd, J = 8.6, 2.9 Hz, 1H), 6.48 (d, J = 2.9 Hz, 1H), 3.70 (s, 3H), 3.49 (s, 2H), 2.29 (s, 3H), 2.16 (s, 3H); [M+H]+ 219.3.
References:
EPIGENETIX, INC.;ALBERT, Jeffrey, S.;JOHNSTONE, Shawn;JONES, Paul WO2014/152029, 2014, A2 Location in patent:Paragraph 00231
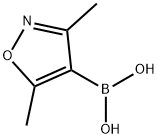
16114-47-9
260 suppliers
$6.00/250mg

74587-12-5
126 suppliers
$9.00/1g
![[3-(3,5-diMethyl-4-isoxazolyl)-4-(Methyloxy)phenyl]aMine](/CAS/20150408/GIF/1300031-61-1.gif)
1300031-61-1
1 suppliers
inquiry
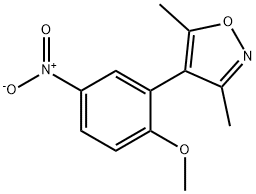
1300031-62-2
1 suppliers
inquiry
![[3-(3,5-diMethyl-4-isoxazolyl)-4-(Methyloxy)phenyl]aMine](/CAS/20150408/GIF/1300031-61-1.gif)
1300031-61-1
1 suppliers
inquiry

16114-47-9
260 suppliers
$6.00/250mg
![[3-(3,5-diMethyl-4-isoxazolyl)-4-(Methyloxy)phenyl]aMine](/CAS/20150408/GIF/1300031-61-1.gif)
1300031-61-1
1 suppliers
inquiry
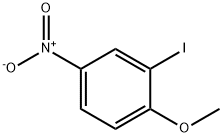
5399-03-1
135 suppliers
$8.00/1g
![[3-(3,5-diMethyl-4-isoxazolyl)-4-(Methyloxy)phenyl]aMine](/CAS/20150408/GIF/1300031-61-1.gif)
1300031-61-1
1 suppliers
inquiry