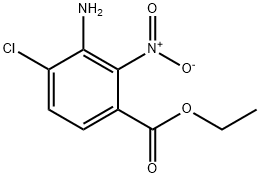
Benzoic acid, 3-amino-4-chloro-2-nitro-, ethyl ester synthesis
- Product Name:Benzoic acid, 3-amino-4-chloro-2-nitro-, ethyl ester
- CAS Number:1277132-56-5
- Molecular formula:C9H9ClN2O4
- Molecular Weight:244.63
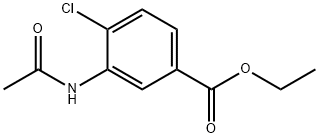
284665-86-7
0 suppliers
inquiry

1277132-56-5
15 suppliers
inquiry
Yield:1277132-56-5 30%
Reaction Conditions:
Stage #1: ethyl 3-acetamido-4-chlorobenzoatewith nitric acid at -15 - 25; for 14 h;
Stage #2: with sulfuric acid in ethanol; for 16 h;Reflux;
Steps:
24.A Step A:
To ethyl 3-acetamido-4-chlorobenzoate (20.0 g, 82.97 mmol) was dropwise added 40.0 mL of 100% HNO3 at -15 °C and the resultant reaction mixture was stirred and warmed up slowly to 10°C during 2 h and then stirred at RT for 12 h, poured into crashed ice, the solids were filtered, dried under reduced pressure and the mixture of nitro compounds (16 g) was used directly in the next step. To a stirred solution of nitro compounds in 160 mL of ethanol was added 7.5 mL of cone. H2SO4. The reaction mixture was refluxed for 16 h, concentrated under reduced pressure and ice- cold water was added. The product was extracted into DCM, the combined organic layers were washed with brine, dried over Na2SC>4 and concentrated. The crude product was purified by flash column chromatography to give ethyl 3-amino-4-chloro-2-nitrobenzoate (6.3 g, 30%).
References:
WO2021/105334,2021,A1 Location in patent:Page/Page column 64; 118
2840-28-0
291 suppliers
$6.00/5g

1277132-56-5
15 suppliers
inquiry
101870-46-6
1 suppliers
inquiry

1277132-56-5
15 suppliers
inquiry