Alectinib Hydrochloride synthesis
- Product Name:Alectinib Hydrochloride
- CAS Number:1256589-74-8
- Molecular formula:C30H35ClN4O2
- Molecular Weight:519.09

1256580-46-7
314 suppliers
$35.00/5mg

1256589-74-8
172 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogenchloride;acetic acid in water;butanone at 60; for 0.5 h;
Steps:
30 Compound F6-20
Hydrochloride Salt
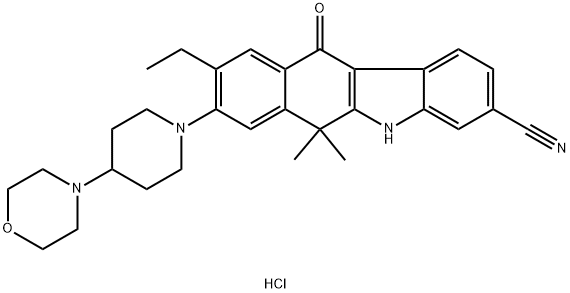
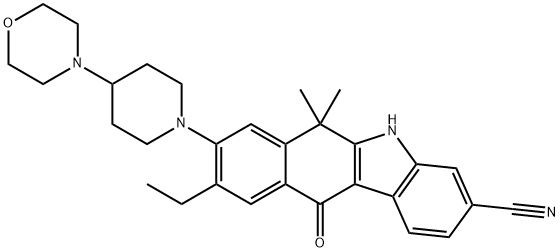
9-Ethyl-6,6-dimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile was dissolved in a mixture of 10 v/w of methyl ethyl ketone, 4 v/w of water, and 3 v/w of acetic acid at 60° C.
To the dissolved solution, 1 v/w of hydrochloric acid (2 N) was added dropwise.
After stirring at 60° C. for 30 minutes, 25 v/w of ethanol was added dropwise.
The precipitated solid was filtered and dried to give 9-ethyl-6,6-dimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile monohydrochloride salt.
9-Ethyl-6,6-dimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile monohydrochloride salt obtained was pulverized by using a jet mill.
1H-NMR (400 MHz, DMSO-D6) δ: 12.78 (1H, s), 10.57 (1H, br.s), 8.30 (1H, J=8.4 Hz), 8.05 (1H, s), 7.99 (1H, s), 7.59 (1H, d, J=7.9 Hz), 7.36 (1H, s), 4.02-3.99 (2H, m), 3.84-3.78 (2H, m), 3.51-3.48 (2H, m), 3.15-3.13 (1H, s), 2.83-2.73 (2H, s), 2.71-2.67 (2H, s), 2.23-2.20 (2H, m), 1.94-1.83 (2H, m), 1.75 (6H, s), 1.27 (3H, t, J=7.5 Hz)
FABMS: m/z 483 [M+H]+
References:
US2013/143877,2013,A1 Location in patent:Paragraph 0683; 0684; 0685
![9-broMo-3-cyano-6,6-diMethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yl trifluoroMethanesulfonate](/CAS/20150408/GIF/1256579-48-2.gif)
1256579-48-2
4 suppliers
inquiry

1256589-74-8
172 suppliers
inquiry
![9-broMo-8-hydroxy-6,6-diMethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile](/CAS/20150408/GIF/1256579-06-2.gif)
1256579-06-2
28 suppliers
inquiry

1256589-74-8
172 suppliers
inquiry
![9-broMo-6,6-diMethyl-8-(4-Morpholinopiperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile](/CAS/20150408/GIF/1256579-62-0.gif)
1256579-62-0
6 suppliers
inquiry

1256589-74-8
172 suppliers
inquiry
![9-ethynyl-6,6-diMethyl-8-(4-Morpholinopiperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile](/CAS/20150408/GIF/1256580-24-1.gif)
1256580-24-1
5 suppliers
inquiry

1256589-74-8
172 suppliers
inquiry