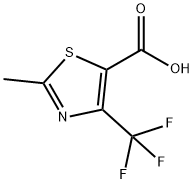
2-METHYL-4-(TRIFLUOROMETHYL)-1,3-THIAZOLE-5-CARBOXYLIC ACID synthesis
- Product Name:2-METHYL-4-(TRIFLUOROMETHYL)-1,3-THIAZOLE-5-CARBOXYLIC ACID
- CAS Number:117724-63-7
- Molecular formula:C6H4F3NO2S
- Molecular Weight:211.16
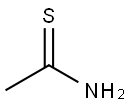
62-55-5
546 suppliers
$9.00/5G

372-31-6
473 suppliers
$5.00/10g

117724-63-7
185 suppliers
$7.00/250mg
Yield:117724-63-7 90.8%
Reaction Conditions:
Stage #1: thioacetamide;ethyl 4,4,4-trifluoroacetoacetate in acetonitrile at 30; for 2.5 h;Large scale;
Stage #2: with tri-n-propylamine in acetonitrile at 30 - 40; for 4 h;Large scale;Further stages;Reagent/catalyst;
Steps:
1 Example 1
(1) into a chain130 kg of acetonitrile into the reactor,32 kg of thioacetamide was added with stirring,90 kg of ethyl trifluoroacetoacetate was added dropwise at 30 ° C,Drop finished,Continue to maintain the chain reaction for 2.5 hours. (2) to form a ringAfter the completion of the chain reaction,120 kg of tri-n-propylamine was added dropwise at 30 ° C,Dropping time for 1 hour,Drop finished,The temperature was raised to 70 ° C for 3 hours to form a ring-forming reaction. (3) hydrolysisAfter the ring-forming reaction,The acetonitrile, water and tri-n-propylamine were separated by vacuum distillation,Respectively, with water acetonitrile fraction and tri-n-propylamine fraction. After acetonitrile and tri-n-propylamine were removed,The reaction solution was cooled to 30 ° C,Add 45% NaOH alkaline solution 93kg,The temperature was raised to 55 ° C for 1 hour to carry out hydrolysis reaction.(4) acidificationAfter the hydrolysis reaction is complete,Cooling to 40 ,30% hydrochloric acid was added dropwise for acidification,Transferred to pH = 1-2, and then continue to cool to 0 ,Add 200kg of water,Stirring for 0.5 hours,Filter,Centrifugal,The filter cake is dried and dried2-methyl-4-trifluoromethyl-5-thiazolecarboxylic acid 82.0 kg,Content of 95.3%,Yield 90.8% (based on ethyl trifluoroacetoacetate).The raw materials used in the preparation are commercially available.
References:
CN106349183,2017,A Location in patent:Paragraph 0029-0047
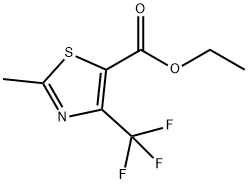
117724-62-6
62 suppliers
$60.00/5mg

117724-63-7
185 suppliers
$7.00/250mg

372-31-6
473 suppliers
$5.00/10g

75-05-8
981 suppliers
$10.00/10g

117724-63-7
185 suppliers
$7.00/250mg
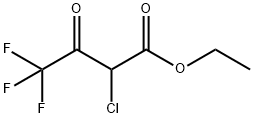
363-58-6
157 suppliers
$10.00/250mg

62-55-5
546 suppliers
$9.00/5G

117724-63-7
185 suppliers
$7.00/250mg