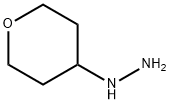
Tetrahydro-pyran-4-yl)-hydrazine synthesis
- Product Name:Tetrahydro-pyran-4-yl)-hydrazine
- CAS Number:116312-69-7
- Molecular formula:C5H12N2O
- Molecular Weight:116.16
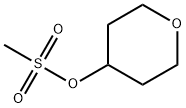
134419-59-3
106 suppliers
$6.00/1g

116312-69-7
47 suppliers
inquiry
Yield: 51%
Reaction Conditions:
with hydrazine in ethanol at 70 - 80; for 3 - 8 h;Product distribution / selectivity;
Steps:
1.1; 14
To a flask having an inner volume of 500 ml, made of glass and equipped with a stirring device, a thermometer and a reflux condenser were charged 134.7 g (710 mmol) of tetrahydropyranyl-4-methanesulfonate with a purity of 95%, 256 ml (5.27 mol) of hydrazine monohydrate and 256 ml of ethanol, and the mixture was reacted at 70 to 80°C for 3 hours under nitrogen atmosphere with stirring. After completion of the reaction, the reaction mixture was cooled to room temperature, 98 ml (784 mmol) of 8 mol/l aqueous sodium hydroxide solution was added to the mixture, and the resulting mixture was concentrated under reduced pressure. After adding 500 ml of toluene to the concentrate, the mixture was filtered, and the filtrate was again concentrated under reduced pressure. Precipitated solid was removed by filtration to obtain 45.0 g (Isolation yield: 51%) of 4-hydrazinotetrahydropyran with a purity of 93% (areal percentage by gas chromatography) as yellowish liquid. Physical properties of the 4-hydrazinotetrahydropyran are as follows. CI-MS(m/e); 117(M+1) 1H-NMR (CDCl3, δ (ppm)); 1.28 to 1.41 (2H, m), 1.84 to 1.90 (2H, m), 2.69 to 2.78 (1H, m), 3.40 to 3.45 (2H, m), 3.92 to 3.98 (2H, m), 4.80 (3H, brs) ; To a flask having an inner volume of 500 ml, made of glass and equipped with a stirring device, a thermometer, a dropping funnel and a reflux condenser were charged 97 ml (1.99 mol) of hydrazine monohydrate and 33 ml of ethanol, and the mixture was heated up to 75°C with stirring. Then, a solution in which 30.0 g (0.124 mol) of 2-tetrahydropyranyl-4-methanesulfonate with a purity of 85% had been dissolved in 33 ml of ethanol was gradually added dropwise thereto, and the mixture was reacted at the same temperature for 8 hours with stirring. After completion of the reaction, the mixture was cooled up to room temperature to obtain a reaction solution containing 4-hydrazinotetrahydropyran as a main product
References:
Ube Industries, Ltd. EP1661894, 2006, A1 Location in patent:Page/Page column 7; 12
693287-79-5
77 suppliers
inquiry

116312-69-7
47 suppliers
inquiry
29943-42-8
423 suppliers
$5.00/1g

116312-69-7
47 suppliers
inquiry
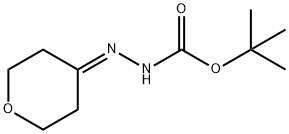
693287-78-4
18 suppliers
inquiry

116312-69-7
47 suppliers
inquiry
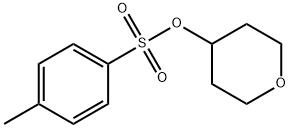
97986-34-0
58 suppliers
$28.00/100mg

116312-69-7
47 suppliers
inquiry