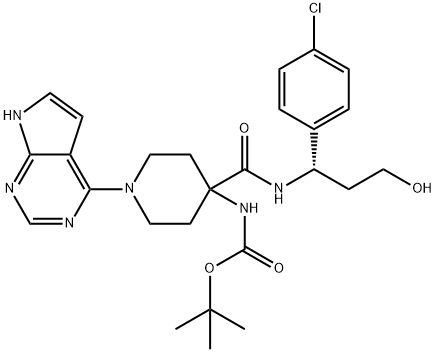
Carbamic acid, N-[4-[[[(1S)-1-(4-chlorophenyl)-3-hydroxypropyl]amino]carbonyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-4-piperidinyl]-, 1,1-dimethylethyl ester synthesis
- Product Name:Carbamic acid, N-[4-[[[(1S)-1-(4-chlorophenyl)-3-hydroxypropyl]amino]carbonyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-4-piperidinyl]-, 1,1-dimethylethyl ester
- CAS Number:1143534-12-6
- Molecular formula:C26H33ClN6O4
- Molecular Weight:529.03
![Benzenepropanoic acid, 4-chloro-β-[[[4-[[(1,1-dimethylethoxy)carbonyl]amino]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-4-piperidinyl]carbonyl]amino]-, methyl ester, (βS)-](/CAS/20210305/GIF/1143534-10-4.gif)
1143534-10-4
1 suppliers
inquiry
![Carbamic acid, N-[4-[[[(1S)-1-(4-chlorophenyl)-3-hydroxypropyl]amino]carbonyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-4-piperidinyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1143534-12-6.gif)
1143534-12-6
3 suppliers
inquiry
Yield:1143534-12-6 86%
Reaction Conditions:
Stage #1: (S)-methyl 3-(4-(tert-butoxycarbonylamino)-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamido)-3-(4-chlorophenyl)propanoatewith lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20; for 1 h;
Stage #2: with sodium hydroxide;water in tetrahydrofuran;Product distribution / selectivity;
Steps:
Intermediate 22: (S)-tert-butyl 4-Q-f4-chlorophenyl)-3-hvdroxypropylcarbamoyl)-l- (7H-pyrrolo [2,3-dl pyrimidin-4- yl)piperidin-4-ylcarbamateLiAlH4 (2.280 mL, 2.28 mmol) was added dropwise to (S)-methyl 3-(4-(tert- butoxycarbonylamino)- 1 -(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamido)-3- (4-chlorophenyl)propanoate (Intermediate 21) (1.27g, 2.28 mmol) in THF (70 mL) cooled to O0C under nitrogen. The resulting solution was stirred at 20 0C for 1 hour. The reaction mixture was quenched with sodium hydroxide (2M) (2mL) and water (ImL). The solution was filtered and was diluted with EtOAc (200 mL), and washed sequentially with water (100 mL), water (100 mL), and saturated brine (100 mL). The organic layer was dried over MgSO4, filtered and evaporated to afford (S)-tert-butyl 4-(l-(4-chlorophenyl)-3- hydroxypropylcarbamoyl)-l-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidin-4-ylcarbamate (1.04g, 86%) as a white solid. M/z (ESI+) (M+H)+ = 529; HPLC tR = 2.00 min.
References:
WO2009/47563,2009,A1 Location in patent:Page/Page column 98