
10-UNDECEN-1-OL synthesis
- Product Name:10-UNDECEN-1-OL
- CAS Number:112-43-6
- Molecular formula:C11H22O
- Molecular Weight:170.29
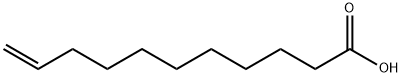
112-38-9
439 suppliers
$5.00/10g

112-43-6
268 suppliers
$5.00/1g
Yield:112-43-6 97%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20; for 0.5 h;Inert atmosphere;
Steps:
Synthesis of 10-undecene-1-ol (3):
To a stirred solution of LiAlH4 (1.50 g, 40.76 mmol) in dry THF (100 mL) was added dropwise 10-undecenoic acid (5.0 g, 27.17 mmol) in dry THF (10 mL) with vigorous stirring at 0 °C and the mixture was stirred for 30 min at room temperature. The reaction mixture was quenched with ethyl acetate. The solution was filtered and the organic layer was washed with H2O, brine and dried over Na2SO4. After removal of the solvent, the residue was purified by column chromatography (4% ethyl acetate in hexane) afforded (3) as a colorless oil (4.48 g, 97%). Spectroscopic data were consistent with the literature data.11 IR (neat) max: 3370, 3076, 2926, 1639, 1460, 1054, 908 cm-1; 1H NMR (300 MHz, CDCl3) : 1.24-1.41 (12H, m, H-3, H-4, H-5, H-6, H-7, H-8), 1.49-1.59 ( 2H, m, H-2), 1.98-2.07 (2H, m, H-9), 3.59 (2H, t, J = 6.79 Hz, H-1), 4.87-4.99 (2H, m, H-11), 5.68-5.83 (1H, m, H-10); m/z ( EI) 152 [M+-18].
References:
Vijai Kumar Reddy;Prabhavathi Devi;Prasad;Poornima;Ganesh Kumar [Bioorganic and Medicinal Chemistry Letters,2012,vol. 22,# 14,p. 4678 - 4680] Location in patent:supporting information; experimental part
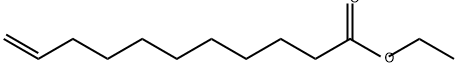
692-86-4
108 suppliers
$20.71/25G

112-43-6
268 suppliers
$5.00/1g

112-45-8
219 suppliers
$8.00/5g

112-43-6
268 suppliers
$5.00/1g

2774-84-7
115 suppliers
$42.70/1g

112-43-6
268 suppliers
$5.00/1g

111-81-9
145 suppliers
$14.00/25g

112-43-6
268 suppliers
$5.00/1g