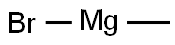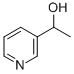
1-PYRIDIN-3-YL-ETHANOL synthesis
- Product Name:1-PYRIDIN-3-YL-ETHANOL
- CAS Number:4754-27-2
- Molecular formula:C7H9NO
- Molecular Weight:123.15
Yield: 76%
Reaction Conditions:
Stage #1:3-pyridinecarboxaldehyde;methylmagnesium bromide in tetrahydrofuran at 0; for 1 h;Inert atmosphere;
Stage #2: with ammonium chloride in tetrahydrofuran;water
Steps:
8-1
REFERENCE EXAMPLE 8-13-(1-hydroxyethyl)pyridine (Compound CA); To methylmagnesium bromide (a 0.96 mol/L solution in tetrahydrofuran, 16.6 mL, 15.9 mmol), a solution of 3-pyridinecarboxaldehyde (500 μL, 5.30 mmol) in tetrahydrofuran (5.0 mL) was slowly added under a nitrogen atmosphere at 0° C., and the mixture was stirred at the same temperature for 1. Then, a saturated aqueous ammonium chloride solution was added to the reaction mixture, and extraction with ethyl acetate was performed, followed by washing with brine and drying over anhydrous sodium sulfate. The solvent was evaporated off under reduced pressure, and the residue was purified by silica gel column chromatography (chloroform/methanol 9/1) to give 3-(1-hydroxyethyl)pyridine (Compound CA) (498 mg, yield: 76%).ESIMS m/z: 124 (M+H)+; 1H-NMR (300 MHz, CDCl3, δ): 1.53 (d, J=6.2 Hz, 3H), 2.62 (br s, 1H), 4.96 (q, J=6.2 Hz, 1H), 7.26-7.30 (m, 1H), 7.74 (ddd, J=1.8, 2.1, 7.7 Hz, 1H), 8.49 (dd, J=1.5, 4.8 Hz, 1H), 8.57 (d, J=1.8 Hz, 1H).
References:
Amishiro, Nobuyoshi;Fukuda, Yuichi;Kinpara, Keisuke;Mie, Motoya;Tagaya, Hisashi;Takahashi, Takeshi US2011/237584, 2011, A1 Location in patent:Page/Page column 131-132

350-03-8
652 suppliers
$10.00/5g

4754-27-2
97 suppliers
$30.00/1g
626-55-1
552 suppliers
$5.00/5 / G
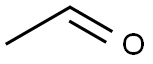
75-07-0
422 suppliers
$14.00/5mL

4754-27-2
97 suppliers
$30.00/1g
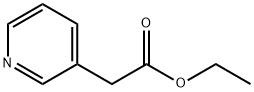
39931-77-6
190 suppliers
$10.00/1g

4754-27-2
97 suppliers
$30.00/1g