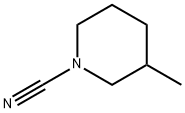
1-Piperidinecarbonitrile,3-methyl-(9CI) synthesis
- Product Name:1-Piperidinecarbonitrile,3-methyl-(9CI)
- CAS Number:150932-26-6
- Molecular formula:C7H12N2
- Molecular Weight:124.18
Yield:150932-26-6 970 mg
Reaction Conditions:
with potassium carbonate in acetone at 0 - 20;
Steps:
15 Synthesis of 3-methylpiperidine-1-carbonitrile (A32):
To a stirred solution of 3-methylpiperidine (1.0 g, 10.08 mmol) in acetone (20 mL) at 0 °C was added K2CO3 (1.67 g, 12.1 mmol) and cyanogen bromide (1.07 g, 10.08 mmol). The reaction mixture was warmed to room temperature and stirred for 2 hours. The reaction mixture was concentrated, treated with water (30 mL), and extracted with ethyl acetate (2 x 50 mL). The organic layer was washed with brine (30 mL), dried over anhydrous Na2SO4, and concentrated to afford A32 (970 mg) as a liquid. The compound was used in the next step without further purification.
References:
WO2022/231872,2022,A1 Location in patent:Paragraph 00174-00175
53152-98-0
1 suppliers
inquiry

150932-26-6
3 suppliers
inquiry
