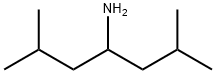
1-isobutyl-3-methylbutylamine synthesis
- Product Name:1-isobutyl-3-methylbutylamine
- CAS Number:65530-92-9
- Molecular formula:C9H21N
- Molecular Weight:143.27

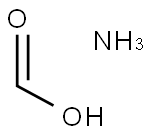
108-83-8
308 suppliers
$16.00/25mL

65530-92-9
9 suppliers
inquiry
Yield:65530-92-9 67%
Reaction Conditions:
Stage #1: diisobutyl ketonewith ammonium acetate in methanol at 20; for 1.5 h;Inert atmosphere;
Stage #2: with sodium cyanoborohydride in methanol at 30;Inert atmosphere;
Steps:
2.4.2.1. Synthesis of 2,6-dimethylheptan-4-amine (iBu2CHNH2).
Ammonium acetate (35.02 g, 454 mmol) was dissolved into MeOH(100 mL) under nitrogen before addition of 10 mL (8.08 g,56.8 mmol) of 2,6-dimethylheptan-4-one. This was stirred for 90 min at room temperature. Sodium cyanoborohydride (2.58 g,41.0 mmol) was dissolved into MeOH (20 mL) and added to the reaction mixture which was left stirring overnight at 30 °C. The reaction vessel was placed in a cold water bath and a liquid nitrogen cooled trap was added. 10 mL of 32% aqueous HCl was added dropwise over the course of 30 min, leading to a highly exothermicreaction and considerable white precipitate. CARE: Hydrolysis of the cyanoborohydride leads to the evolution of hydrogen cyanide which should be caught in the trap and disposed of properly. The MeOH was removed under reduced pressure to give a white slurry.This was extracted with pentane (3 50 mL) and Et2O (3 50 mL)and the solvents again removed to give a clear oil. This was dissolved in Et2O (50 mL) before addition of 5.32 g (133 mmol) of NaOH. The mixture was stirred for 2 h before addition of 5 mL ofwater to form two phases and dissolve the solids. The organic phase was removed and collected and the aqueous phase extracted with Et2O (3 50 mL) and pentane (2 50 mL). The solvent was then removed at reduced pressure. The resulting oil was distilledat 160 °C and 300 mbar to give 5.44 g (38 mmol, 67%) of acolourless non-viscous oil. 1H NMR (CDCl3): d = 2.83 (1H, quintet,J = 6.6, -CHNH2), 1.73 (2H, nonet, J = 6.7, -CHMe2), 1.17 (4H, m,-CH2CHMe2), 0.90 (12H, t, J = 6.4, -CH3). 13C NMR (CDCl3):d = 47.99 (-CH2CHMe2), 46.24 (-CHCH2NH2), 24.44 (-CHMe2),23.24 (-CH3), 21.79 (-CH3).
References:
K?hn, Randolf D.;Coxon, Alexander G.N.;Chunawat, Samaphon;Heron, Callum;Mihan, Shahram;Lyall, Catherine L.;Reeksting, Shaun B.;Kociok-K?hn, Gabriele [Polyhedron,2020,vol. 185,art. no. 114572]
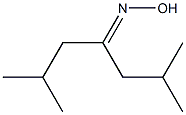
52435-41-3
3 suppliers
inquiry

65530-92-9
9 suppliers
inquiry