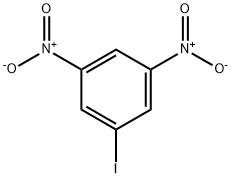
1-IODO-3,5-DINITROBENZENE synthesis
- Product Name:1-IODO-3,5-DINITROBENZENE
- CAS Number:6276-04-6
- Molecular formula:C6H3IN2O4
- Molecular Weight:294
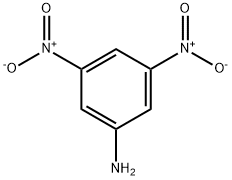
618-87-1
3 suppliers
$90.00/1g

6276-04-6
58 suppliers
$88.51/1G
Yield: 97%
Reaction Conditions:
Stage #1:3, 5-dinitroaniline with sulfuric acid;sodium nitrite at 0;
Stage #2: with potassium iodide at 20; for 3 h;
Steps:
3.2.1. Synthesis of 1-Iodo-3,5-dinitrobenzene (1)
To a stirred mixture of 3,5-dinitroaniline (C6H5N3O4) (0.70 g, 3.82 mmol, 1.0 eq.) and sulfuric acid(H2SO4) (0.60 mL, 11.46 mmol, 3.0 eq.), sodium nitrite (NaNO2) (0.40 g, 5.73 mmol, 1.5 eq.) was addedat 0 C. After the formation of the diazonium salt was conrmed by TLC, potassium iodide (KI) (1.60 g,9.55 mmol, 2.5 eq.) was added to the mixture and the mixture was stirred at room temperature for3 h. After the reaction was completed, pH was adjusted to neutral using saturated aqueous sodiumbicarbonate (NaHCO3). Then the reaction mixture was extracted with ethyl acetate. The organic layerwas separated, dried over sodium sulfate (Na2SO4), filtered and concentrated under reduced pressureto aord 1 (1.1 g, 97%) as a brown crystal. 1H-NMR (400 MHz, CDCl3): 8.88 (2H, d, J = 1.6 Hz), 9.02 (1H, t, J = 2.0 Hz). 13C-NMR (100 MHz, CDCl3): 93.5, 118.5, 137.8, 148.5. HRMS (FAB+) calculatedfor C6H3IN2O4 [M + H]+: m/z = 294.9216, found 294.9225.
References:
Fawwaz, Muammar;Kinuya, Seigo;Mishiro, Kenji;Nishii, Ryuichi;Ogawa, Kazuma;Sawazaki, Izumi;Shiba, Kazuhiro [Molecules,2020,vol. 25,# 12]
610-30-0
129 suppliers
$15.00/5mg

6276-04-6
58 suppliers
$88.51/1G
18242-39-2
144 suppliers
$6.00/250mg

6276-04-6
58 suppliers
$88.51/1G

99-65-0
9 suppliers
$31.39/442244

6276-04-6
58 suppliers
$88.51/1G
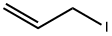
556-56-9
134 suppliers
$10.00/1g

618-87-1
3 suppliers
$90.00/1g

6276-04-6
58 suppliers
$88.51/1G
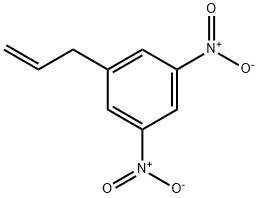
250782-02-6
0 suppliers
inquiry