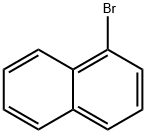
1-Bromonaphthalene synthesis
- Product Name:1-Bromonaphthalene
- CAS Number:90-11-9
- Molecular formula:C10H7Br
- Molecular Weight:207.07
Reaction: add carbon tetrachloride and naphthalene in the reaction pot, stirring and heating, and slowly add bromine at 45℃. After adding, keep the reaction at 70-80℃ for 3-4h. distillation to recover carbon tetrachloride, wash the reaction product, distill under reduced pressure, wash again, filter, dry and get the finished product.
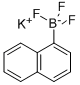
166328-07-0
56 suppliers
$13.00/250mg

90-11-9
515 suppliers
$5.00/10g
Yield:90-11-9 90%
Reaction Conditions:
with pyridinium hydrobromide perbromide in tetrahydrofuran;water at 20; for 0.333333 h;chemoselective reaction;
Steps:
4.2. Typical procedure for bromodeboronation reactions
General procedure: The trifluoroborate (1.0 mmol) was dissolved in (1:1 v/v) mixture of tetrahydrofuran and water (10 mL). Pyridinium tribromide (or tetrabutylammonium tribromide) (1.0 mmol) was added to this solution. The mixture was stirred for the appropriate time and temperature (Tables 1and 2) and then diluted with 10 mL of ether. The aqueous layer was extracted twice with ether (5 mL) and the combined organic phase was dried over anhydrous Na2SO4. After evaporation of the solvent the residue was purified by silica gel column chromatography [elute: hexane (or pentane)-ethyl acetate (or Et2O)].
References:
Yao, Min-Liang;Kabalka, George W.;Blevins, David W.;Reddy, Marepally Srinivasa;Yong, Li [Tetrahedron,2012,vol. 68,# 19,p. 3738 - 3743] Location in patent:experimental part
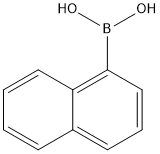
13922-41-3
497 suppliers
$5.00/1g

90-11-9
515 suppliers
$5.00/10g

90-14-2
290 suppliers
$19.00/5g

90-11-9
515 suppliers
$5.00/10g
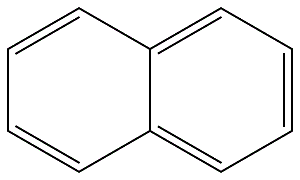
91-20-3
510 suppliers
$14.00/25g

90-11-9
515 suppliers
$5.00/10g

17135-74-9
274 suppliers
$8.00/250mg

90-11-9
515 suppliers
$5.00/10g