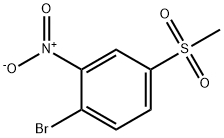
1-BROMO-4-(METHYLSULPHONYL)-2-NITROBENZENE synthesis
- Product Name:1-BROMO-4-(METHYLSULPHONYL)-2-NITROBENZENE
- CAS Number:94832-06-1
- Molecular formula:C7H6BrNO4S
- Molecular Weight:280.1

3466-32-8
198 suppliers
$8.00/1g

94832-06-1
39 suppliers
$45.00/100mg
Yield: 90.5%
Reaction Conditions:
with sulfuric acid;nitric acid at 0 - 10; for 0.5 h;
Steps:
3
Add 120 ml of concentrated nitric acid (65%) to the reaction flask. Stir in an ice bath, add 50 ml of concentrated sulfuric acid (98%) at 0 °C, and add dropwise after 0.5 hours. 23.5 g of p-methanesulfonyl bromide (0.1 mol) was added to the reaction flask, and the reaction was carried out at 0 to 10 ° C for 0.5 hour. After the reaction, the reaction mixture was extracted with 500 ml of a saturated aqueous sodium hydrogen carbonate solution and 1000 ml of ethyl acetate. Collect the organic phase after the extraction, distillation under reduced pressure gave 25.3 g of o-nitro-methanesulfonyl bromide. The yield was 90.5%.
References:
Jiangsu Changqing Agrochemical Co., Ltd.;Yu Guoquan;Sun Xialin;Ma Changqing;Ding Huaping CN109134321, 2019, A Location in patent:Paragraph 0006; 0014; 0015; 0016