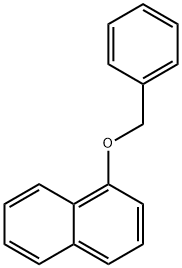
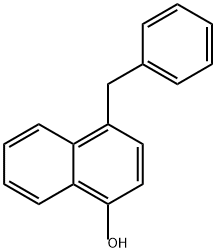
1-Benzyloxynaphthalene synthesis
- Product Name:1-Benzyloxynaphthalene
- CAS Number:607-58-9
- Molecular formula:C17H14O
- Molecular Weight:234.29
Yield:607-58-9 91%
Reaction Conditions:
with potassium carbonate in acetonitrile at 125; for 0.333333 h;Microwave irradiation;Temperature;
Steps:
General Procedure for S-L Phase Alkylation of 1- and 2-Naphthol (1) and (6) under Solventless and MWConditions
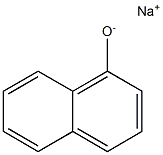
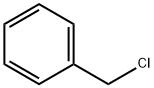
General procedure: A mixture of 0.14 g (1.0 mmol) of 1- and 2-naphthol (1)and (6), in most cases 1.0 mmol of alkali carbonate (0.14 g of K2CO3 or 0.33 g of Cs2CO3), in certain cases 11.4 mg(0.05 mmol) of TEBAC and 1.2 mmol of alkyl halide (0.14ml of benzyl bromide, 0.10 ml of ethyl iodide, 0.12 mol ofbutyl bromide or 0.11 ml of i-propyl bromide) in a closedvial was irradiated (20-30 W) in a CEM Discover [300 W]MW reactor at 125 °C for the appropriate time. The reactionmixture was taken up in 25 ml of ethyl acetate and the suspensionwas filtered. Evaporation of the volatile componentsprovided the crude product that was passed through a thin(ca. 2-3 cm) layer of silica gel using ethyl acetate as theeluant to give an oil that was analysed by GC-MS or GC.Similar reactions were carried out in 3 ml of MeCN asthe solvent. The work-up was similar to that described forthe solventless alkylations above, but in this case, ethyl acetatedid not have to be added.The major components of the above reactions, such ascompounds 2, 7, 8, 10a-c and 13a,b were obtained in a pureform by repeated chromatography.Control experiments were performed with benzyl bromidein a similar way under conventional heating.
References:
Balint, Erika;Kovacs, Orsolya;Drahos, Laszlo;Keglevich, Gyoergy [Letters in Organic Chemistry,2013,vol. 10,# 5,p. 330 - 336]
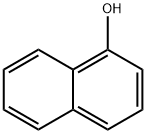
90-15-3
715 suppliers
$9.00/5g
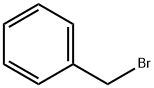
100-39-0
432 suppliers
$10.00/10g
28178-96-3
0 suppliers
inquiry

607-58-9
36 suppliers
$65.00/100mg

90-14-2
288 suppliers
$19.00/5g

100-51-6
1392 suppliers
$5.00/100g

607-58-9
36 suppliers
$65.00/100mg