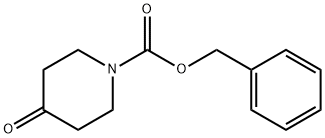
1-Cbz-4-Piperidone synthesis
- Product Name: 1-Cbz-4-Piperidone
- CAS Number:19099-93-5
- Molecular formula:C13H15NO3
- Molecular Weight:233.26

501-53-1
398 suppliers
$10.00/1g

19099-93-5
318 suppliers
$5.00/1g
Yield:19099-93-5 85%
Reaction Conditions:
Stage #1:4-piperidone hydrochloride with N-ethyl-N,N-diisopropylamine in dichloromethane;water at 0; for 0.0833333 h;
Stage #2:benzyl chloroformate in dichloromethane;water at 0 - 20; for 2.33333 h;
Steps:
1
Example 1;. N-Benzyloxycarbonyl-4-oxopiperidine (E-1);. A stirred solution of 4-oxopiperidine hydrochloride monohydrate (1.0 g, 6.5 mmol) in dry dichloromethane (DCM, 40 mL) was cooled to 0°C, treated with diisopropylethylamine (3.40 mL, 19.5 mmol), stirred for five minutes, treated over 20 minutes with benzyl chloroformate (1.54 mL, 10.7 mmol) over 20 minutes, allowed to warm to room temperature and stirred for two hours. The mixture was partitioned between DCM (25 mL) and water (15mL). The layers were separated and the aqueous phase was extracted with DCM (2 x 25 mL). The combined organic phases were washed with brine (1 x 15 mL), dried over Na2S04 and evaporated to a residue that was purified by column chromatography using a gradient of 20 to 40 % EtOAc in hexanes as eluant. Evaporation of the collected fractions gave carbamate E-1 (1.20 g, 85 %) as a clear oil: HRMS calc'd for C13Hl5NO3 (M+) : 233. 1051, found: 233.1048 ; 1H NMR (CDC13) 8 2.43 (s, 4H), 3.78 (d, 4H, J= 5.96), 5.16 (s, 2H), 7. 35 (m, 5H).
References:
BIOAXONE THERAPEUTIQUE INC. WO2005/80394, 2005, A1 Location in patent:Page/Page column 122
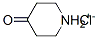
41979-39-9
116 suppliers
$15.00/10mg

501-53-1
398 suppliers
$10.00/1g

19099-93-5
318 suppliers
$5.00/1g
13139-17-8
503 suppliers
$6.00/25g
40064-34-4
366 suppliers
$10.00/5g

19099-93-5
318 suppliers
$5.00/1g
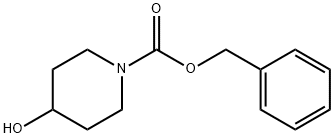
95798-23-5
142 suppliers
$5.00/250mg

19099-93-5
318 suppliers
$5.00/1g
![8-(BENZYLOXYCARBONYL)-1,4-DIOXA-8-AZASPIRO[4.5]DECANE](/CAS/20180601/GIF/139524-58-6.gif)
139524-58-6
12 suppliers
$640.00/5g

19099-93-5
318 suppliers
$5.00/1g