
1-Benzhydrylazetidine-3-carboxylic acid synthesis
- Product Name:1-Benzhydrylazetidine-3-carboxylic acid
- CAS Number:36476-87-6
- Molecular formula:C17H17NO2
- Molecular Weight:267.32
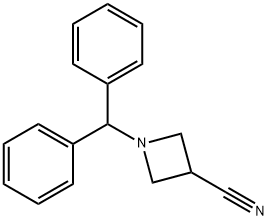
36476-86-5
184 suppliers
$6.00/1g

36476-87-6
195 suppliers
$10.00/1g
Yield:36476-87-6 71.7%
Reaction Conditions:
Stage #1:1-(1,1-diphenylmethyl)azetidine-3-carbonitrile with potassium hydroxide;water in 2-methoxy-ethanol at 100; for 4 h;
Stage #2: with hydrogenchloride in water; pH=5
Steps:
12 1-Benzhydrylazetidine-3-carboxylic acid
Potassium hydroxide (6.48 g) and water (3.25 ml) were added to a solution of 1-benzhydryl-3-cyanoazetidine (5.43 g) in methoxyethanol (54 ml), followed by stirring at 100° C. for 4 hours. The reaction mixture was allowed to cool down to room temperature, and poured into ice. After pH of the mixture was adjusted to 5 with 1N hydrochloric acid, sodium chloride was added thereto. This was extracted with a mixed solvent of ethyl acetate and tetrahydrofuran. The organic layer was washed with brine, and dried over anhydrous sodium sulfate. The dried organic layer was concentrated under reduced pressure to give a crude product of the title compound as pale yellow crystals. The crystals were suspended by the addition of diethyl ether (15 ml). The crystals were collected by filtration and washed with diethyl ether. This was dried under aeration to give the title compound (4.20 g, 71.7%) as pale yellow crystals.1H-NMR Spectrum (CDCl3) δ (ppm): 3.00-3.90 (5H, m), 4.95 (1H, s), 7.25-7.28 (2H, m), 7.33 (4H, m), 7.53 (4H, m).
References:
Eisai R&D Management Co., Ltd. US2008/214815, 2008, A1 Location in patent:Page/Page column 14
53871-06-0
94 suppliers
$38.00/1 g

36476-87-6
195 suppliers
$10.00/1g
18621-17-5
377 suppliers
$5.00/1g

36476-87-6
195 suppliers
$10.00/1g
33301-41-6
232 suppliers
$13.50/250mg

36476-87-6
195 suppliers
$10.00/1g