
1,8-Diaminooctane synthesis
- Product Name:1,8-Diaminooctane
- CAS Number:373-44-4
- Molecular formula:C8H20N2
- Molecular Weight:144.26
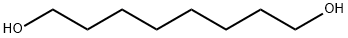
629-41-4
362 suppliers
$9.00/10g

373-44-4
228 suppliers
$6.00/5g
Yield:373-44-4 78%
Reaction Conditions:
with ammonia;carbonylchloro[4,5-bis(diisopropylphosphinomethyl)acridine]hydridoruthenium(II) in tert-Amyl alcohol at 140; for 48 h;Product distribution / selectivity;Autoclave;
Steps:
3
Example 3; Reaction of 1,8-octanediol; The reaction was carried out under the same conditions as in Example 1 using 10.0 mmol of 1,8-octanediol. Yield and conversion were determined by gas chromatography using commercially available reference compounds. Yield of linear primary diamine: 78%, conversion of the linear diol: >99%.; Example 1; Reaction of 1,19-nonadecanediol; 3.01 g (10.0 mmol) of 1,19-nonadecanediol and 151 mg (0.25 mol %) of carbonylchloro[4,5-bis(diisopropylphosphinomethyl)acridine]hydridoruthenium(II) were dissolved under protective gas in 25 ml of 2-methyl-2-butanol and transferred to an autoclave provided with stirrer, heating and temperature measuring facility. 6 ml of liquid ammonia were subsequently introduced into the autoclave by means of a spindle press. The autoclave was closed and the contents stirred at 140° C. for 48 hours. This resulted in an increase in the internal pressure from 22 to 40 bar. After cooling, the contents of the reactor were filtered through kieselguhr, the filtrate was evaporated to dryness and the residue subjected to a bulb tube distillation. Yield of linear primary diamine: 2.02 g (68% of theory; bp0.8 mbar=170-185° C.), conversion of the linear diol: >99%.
References:
Evonik Degussa GmbH US2012/203033, 2012, A1 Location in patent:Page/Page column 4
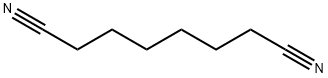
629-40-3
146 suppliers
$28.10/25g

373-44-4
228 suppliers
$6.00/5g
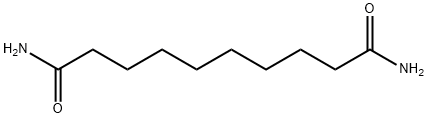
1740-54-1
12 suppliers
inquiry

373-44-4
228 suppliers
$6.00/5g
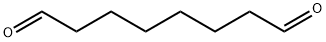
638-54-0
12 suppliers
inquiry

373-44-4
228 suppliers
$6.00/5g
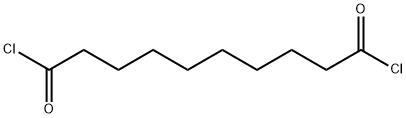
111-19-3
165 suppliers
$14.00/5g

373-44-4
228 suppliers
$6.00/5g