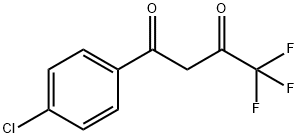
1-(4-CHLOROPHENYL)-4,4,4-TRIFLUORO-1,3-BUTANEDIONE synthesis
- Product Name:1-(4-CHLOROPHENYL)-4,4,4-TRIFLUORO-1,3-BUTANEDIONE
- CAS Number:18931-60-7
- Molecular formula:C10H6ClF3O2
- Molecular Weight:250.6

383-63-1
479 suppliers
$10.00/25g

99-91-2
431 suppliers
$10.00/25g

18931-60-7
99 suppliers
$13.00/500mg
Yield:18931-60-7 86%
Reaction Conditions:
Stage #1: ethyl trifluoroacetate,;para-chloroacetophenonewith sodium methylate in methanol;tert-butyl methyl ether; for 16 h;Heating / reflux;
Stage #2: with hydrogenchloride in methanol;tert-butyl methyl ether;water;
Steps:
4-[5-(4-Chlorophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzene-carboxyamide
The title compound was synthesized via a two-step synthesis. Step a. The preparation of 4,4,4-trifluoro-1-(4-chlorophenyl)butane-1,3-dione was carried out as follows. To a solution of ethyl trifluoroacetate (1.08 g, 7.61 mmol) in 5 mL of methyl tert-butyl ether (MTBE) was added 25% sodium methoxide in methanol (1.8 mL)2 min. A solution of 4'-chloroacetophenone (1 g, 6.46 mmol) in 2 mL MTBE was added to the mixture dropwise5 min. After stirring for 16 h, 3 N HCl (3.4 mL) was added. The organic layer was collected, washed with brine, driedmagnesium sulfate, and concentrated to give a yellow-orange solid. Recrystallization from hexane yielded the dione (1.18 g, 86%). Step b. (4-Carbamoylphenyl)hydrazine hydrochloride (228 mg, 1.21 mmol) was added to a stirred solution of the aforementioned dione (300 mg, 1.21 mmol) in 20 mL of ethanol. The mixture was stirred under reflux for 24 h, cooled to room temperature, and concentrated to dryness. The residue was dissolved in ethyl acetate, washed with brine, driedmagnesium sulfate, and concentrated to give a light brown solid. Recrystallization from ethyl acetate and hexane gave the title compound (350 mg, 80%): 1H NMR (CDCl3) δ {tilde()}s, 1H), 7.16 (d, J=8.5 Hz, 2H), 7.34 (d, J=8.4 Hz, 2H), 7.40 (d, J=8.4 Hz, 2H), 7.83 (d, J=8.4 Hz, 2H); HRMS calc'd for M+ 365.0535, found 365.0522. Anal. (C17H11ClF3N3O)C, H, N.
References:
US2003/236294,2003,A1 Location in patent:Page 8, 18

431-47-0
283 suppliers
$10.00/5g

99-91-2
431 suppliers
$10.00/25g

18931-60-7
99 suppliers
$13.00/500mg