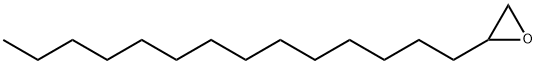
1,2-EPOXYHEXADECANE synthesis
- Product Name:1,2-EPOXYHEXADECANE
- CAS Number:7320-37-8
- Molecular formula:C16H32O
- Molecular Weight:240.42

629-80-1
124 suppliers
$35.00/100mg

7320-37-8
88 suppliers
$20.00/5 g
Yield:7320-37-8 81%
Reaction Conditions:
Stage #1:n-hexadecylaldehyde with N-chloro-succinimide;rac-Pro-OH in dichloromethane at 20;
Stage #2: with sodium tetrahydroborate in ethanol;dichloromethane at 0;
Stage #3: with potassium hydroxide in ethanol;dichloromethane;water at 20; for 0.5 h;
Steps:
3.2.2. General Procedure for the Synthesis of Racemic Epoxides 6, 15
General procedure: To a solution of 5 or 14 (1.00 mmol) in CH2Cl2 (5 mL), d,l-proline (115 mg, 0.10 mmol) andN-chlorosuccinimide (174 mg, 1.30 mmol) were added and the reaction mixture was stirred for 16 h at room temperature. The reaction mixture was cooled to 0 °C, before NaBH4 (121 mg, 3.20 mmol)and EtOH (1 mL) were added. After 10 min, the medium was warmed to room temperature for 5 min before a freshly prepared solution of aqueous KOH (2.25 g KOH diluted in 3.4 mL distilled water)and EtOH (1.7 mL) were added and the resulting mixture was stirred vigorously for 30 min. At thisstage, water (10 mL) was added to the mixture before it was extracted with Et2O (1 10 mL), saturatedaqueous solution of NH4Cl (1 x 10 mL) and brine (1 x 10 mL). The combined organic layers were driedover Na2SO4 and concentrated in vacuo. The resulting residue purified by column chromatography (petroleum ether 40-60 °C/diethyl ether, 9:1 or 8:2).
References:
Bourboula, Asimina;Limnios, Dimitris;Kokotou, Maroula G.;Mountanea, Olga G.;Kokotos, George [Molecules,2019,vol. 24,# 11,art. no. 2081]
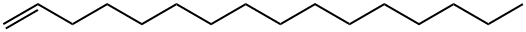
629-73-2
249 suppliers
$5.00/5g

7320-37-8
88 suppliers
$20.00/5 g
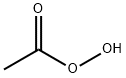
79-21-0
2 suppliers
$62.79/5ml
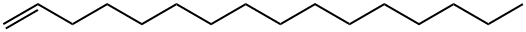
629-73-2
249 suppliers
$5.00/5g
64-19-7
1553 suppliers
$10.00/25ML

7320-37-8
88 suppliers
$20.00/5 g