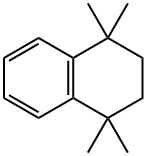
1,1,4,4-Tetramethyl-1,2,3,4-tetrahydronaphthalene synthesis
- Product Name:1,1,4,4-Tetramethyl-1,2,3,4-tetrahydronaphthalene
- CAS Number:6683-46-1
- Molecular formula:C14H20
- Molecular Weight:188.31
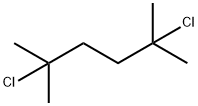
6223-78-5
128 suppliers
$9.00/5g

71-43-2
694 suppliers
$11.19/25ML

6683-46-1
116 suppliers
$9.00/100mg
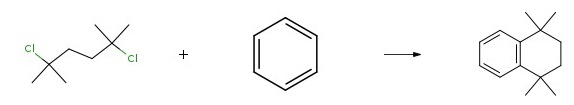
Yield: 91%
Reaction Conditions:
with aluminum (III) chloride for 16 h;Reflux;
Steps:
2 l,l,4,4-tetramethyl-l,2,3,4-tetrahydronaphth.alene (18):
l,l,4,4-tetramethyl-l,2,3,4-tetrahydronaphth.alene (18): 2,5-dichloro-2,5- dimethylhexane (300 mg, 1.64 mmol, 1 eq) was dissolved in dry benzene (25 mL). A1C13 (22.0 mg, 0.164 mmol, 0.1 eq) was added to the solution, which was stirred at reflux for 16 hours. The reaction was quenched with 3M HC1 (5 mL) and extracted with hexanes (10 mL x 3). The combined organic layer was washed with brine, dried with Na2S04, filtered, and concentrated in vacuo. The crude was purified by flash chromatography (100% hexanes) to give 18 (309 mg, 91% yield) as a colourless oil. Characterization data for 18: <5H (500MHz, CDC13) 1.33 (s, 12H), 1.74 (s, 4H), 7.16-7.19 (m, 2H), 7.34-7.36 (m, 2H, J 2 Hz and 8.4 Hz).<5c (500MHz, CDC13) 31.9, 34.2, 35.1, 125.5, 126.5, 144.8.
References:
HARO PHARMACEUTICAL INC.;THE ROYAL INSTITUTION FOR THE ADVANCEMENT OF LEARNING/MCGILL UNIVERSITY;UNIVERSITE DE MONTREAL;BETTOUN, David;MARTINEZ, Eduardo, J.;GLEASON, James;MADER, Sylvie;XING, Shuo WO2015/188015, 2015, A1 Location in patent:Page/Page column 31

6223-78-5
128 suppliers
$9.00/5g

6683-46-1
116 suppliers
$9.00/100mg

110-03-2
350 suppliers
$6.00/25g

6683-46-1
116 suppliers
$9.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

6683-46-1
116 suppliers
$9.00/100mg

110-03-2
350 suppliers
$6.00/25g

71-43-2
694 suppliers
$11.19/25ML

6683-46-1
116 suppliers
$9.00/100mg
