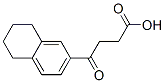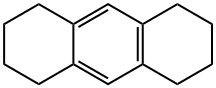
1,2,3,4,5,6,7,8-OCTAHYDROANTHRACENE synthesis
- Product Name:1,2,3,4,5,6,7,8-OCTAHYDROANTHRACENE
- CAS Number:1079-71-6
- Molecular formula:C14H18
- Molecular Weight:186.29
Yield:-
Reaction Conditions:
with Dimethylphenylsilane;iodine;indium(III) acetate in chloroform at 60; for 5 h;Inert atmosphere;Sealed tube;Overall yield = 86 %; Overall yield = 96.2 mg;
Steps:
General preparation of tetralin derivatives:
General procedure: Freshly distilled chloroform (0.6 mL)was placed into a screw-capped vial under N2, and a magnetic stirrer bar, In(OAc)3(0.0060 mmol, 1.8 mg), I2 (0.600 mmol, 152 mg), a carboxylic acid (0.6 mmol), andMe2PhSiH (3.90 mmol, 600 μL) were successively added. The vial was sealed with acap that contained a PTFE septum. The reaction vial was heated in an oil bath, and thereaction was monitored by GC and TLC until the ester had been completely consumed.After the reaction was complete, the mixture was cooled to room temperature, and wasquenched with HCl aqueous solution (2 mL). The aqueous layer was extracted withhexane (5 mL x 3). The combined organic phases were dried with anhydrous Na2SO4,and the solvent was evaporated under reduced pressure. The crude product was purifiedby silica gel column chromatography (hexane) to give the corresponding tetralinderivatives. If necessary, further purification was performed by gel permeationchromatography (eluent: CHCl3).
References:
Sakai, Norio;Kobayashi, Taichi;Ogiwara, Yohei [Chemistry Letters,2015,vol. 44,# 11,p. 1503 - 1505] Location in patent:supporting information

120-12-7
328 suppliers
$10.00/5g

1079-71-6
19 suppliers
$144.00/1g

120-12-7
328 suppliers
$10.00/5g
613-31-0
125 suppliers
$11.00/1g
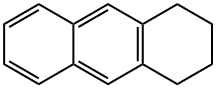
2141-42-6
6 suppliers
inquiry

1079-71-6
19 suppliers
$144.00/1g
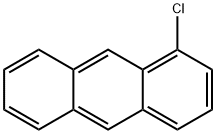
4985-70-0
25 suppliers
$142.00/1g

1079-71-6
19 suppliers
$144.00/1g