
1,1-DIMETHOXYCYCLOHEXANE synthesis
- Product Name:1,1-DIMETHOXYCYCLOHEXANE
- CAS Number:933-40-4
- Molecular formula:C8H16O2
- Molecular Weight:144.21

108-94-1
539 suppliers
$12.00/50g

122-51-0
488 suppliers
$10.00/5ml

504-63-2
536 suppliers
$9.00/5g

933-40-4
113 suppliers
$22.39/5ML
Yield:933-40-4 55%
Reaction Conditions:
Stage #1:cyclohexanone;orthoformic acid triethyl ester;trimethyleneglycol with zirconium(IV) chloride in dichloromethane at 20; for 1 h;
Stage #2: with sodium hydroxide
Steps:
14 REFERENCE EXAMPLE 14 4-Cyclohexyloxybut-1-yne
To anhydrous dichloromethane (950 ml) were added cyclohexanone (32 ml, 0.31 mol), 1, 3-propanediol (33.5 ml, 0.46 mol), triethyl orthoformate (51.5 ml, 0.31 mol), and zirconium chloride (1.44 g, 6.18 mmol) followed by stirring under a nitrogen atmosphere for 1 hour at room temperature. An ice-cold 1N aqueous sodium hydroxide solution (1.51) was added to the reaction mixture, and the reaction solution was extracted with dichloromethane, and then the dichloromethane layer was washed with water. The dichloromethane layer was dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by distillation under reduced pressure to give cyclohexanone trimethyl ketal (26.8 g, 55% yield). To the suspension of zirconium chloride (24.9 g, 0.11 mol) in tetrahydrofuran (500 ml) was slowly added sodium borohydride (20.5 g, 0.54 mmol) under a nitrogen atmosphere followed by stirring for 20 minutes at room temperature. A solution of tetrahydrofuran (170 ml) including cyclohexanone trimethyl ketal (16.9 g, 0.11 mol) obtained above was added dropwise in a nitrogen atmosphere to the reaction solution in an ice bath. After the end of dropping, the reaction solution was stirred overnight at room temperature. To this reaction solution was added ice-cold 2N hydrochloric acid (600 ml) in the ice bath to stop the reaction, and tetrahydrofuran was evaporated in vacuo. The water layer residue was extracted with ethyl acetate, and the ethyl acetate layer was washed with a saturated aqueous sodium chloride solution. The ethyl acetate layer was dried over anhydrous sodium sulfate, and the solvent was evaporated in vacuo. The obtained residue was purified by chromatography on a silica gel column (elution solvent; hexane:ethyl acetate=10:1-5:2) to afford 3-cyclohexyloxypropan-1-ol (13.4 g, 78% yield). [1401] The obtained 3-cyclohexyloxypropan-1-ol (11.5 g, 72.9 mmol) was dissolved in dichloromethane (240 ml), and then molecular sieve 4 (58 g) and pyridinium chlorochromate (23.8 g, 0.11 mol) were added thereto in the ice bath followed by stirring under a nitrogen atmosphere for 1 hour and 40 minutes. The reaction solution was diluted with ether, and then the solution was filtered through Celite. The Celite was washed with diethyl ether, and this filtrate was added to the previous filtrate. The total filtrate was evaporated in vacuo, and the residue was purified roughly by chromatography on a silica gel column (elution solvent; n-hexane:ethyl acetate=20:10:1) to give a crude 3-cyclohexyloxypropionaldehyde (8.6 g). [1402] A dichloromethane solution (120 ml) of triphenylphosphine (57.7 g, 0.22 mol) in an ice bath were added dropwise to the dichloromethane solution (120 ml) including carbon tetrabromide (36.5 g, 0.11 mol) under a nitrogen atmosphere. After the end of dropping, the reaction mixture was stirred for 5 more minutes. To the reaction solution in the ice bath was added dropwise under a nitrogen atmosphere a dichloromethane solution (90 ml) of the crude 3-cyclohexyloxypropionaldehyde (8.6 g) obtained above, and after dropping, the reaction mixture was stirred for 25 more minutes. The reaction solution was diluted with dichloromethane, and washed with a saturated aqueous sodium hydrogencarbonate solution and then with a saturated aqueous sodium chloride solution. After the dichloromethane layer was dried over anhydrous sodium sulfate, the solvent was evaporated in vacuo. The residue was purified by chromatography on a silica gel column (elution solvent; hexane:ethyl acetate=100:1-33:1) to afford 4-cyclohexyloxy-1,1-dibromobut-1-ene (12.6 g, 55% yield, 2 processes). [1403] To a tetrahydrofuran solution (130 ml) of 4-cyclohexyloxy-1,1-dibromobut-1-ene (12.6 g, 40.4 mmol) obtained above, was added dropwise under a nitrogen atmosphere at -78° C. the hexane solution of 1.5N n-butyllithium (54 ml, 81.0 mmol). After the end of dropping, the reaction solution was stirred for 1 hour, and then warmed up gradually to room temperature. After the reaction solution was stirred at room temperature for 50 minutes, water was added thereto in the ice bath to terminate the reaction. The resulting reaction solution was extracted with diethyl ether, and the diethyl ether layer was washed with the saturated aqueous sodium chloride solution. The diethyl ether layer was dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by chromatography on a silica gel column (elution solvent; hexane:ethyl acetate=100:1-50:1) to give the title compound (4.35 g, 71% yield). [1404] Nuclear magnetic resonance spectrum (400 MHz, CDCl3) δ ppm: 1.13-1.36 (5H, m), 1.48-1.58 (1H, m), 1.67-1.81 (2H, m), 1.85-1.95 (2H, m), 1.97 (1H, t, J=2.8 Hz), 2.45 (2H, dt, J=2.8, 7.2 Hz), 3.23-3.32 (1H, m), 3.59 (2H, t, J=7.2 Hz) [1405] Mass spectrum (El) m/z: 153 (M+H)+
References:
SANKYO COMPANY, LIMITED US2003/236297, 2003, A1 Location in patent:Page 117

108-94-1
539 suppliers
$12.00/50g
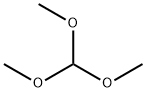
149-73-5
525 suppliers
$12.00/5g

933-40-4
113 suppliers
$22.39/5ML

67-56-1
746 suppliers
$7.29/5ml-f

108-94-1
539 suppliers
$12.00/50g

933-40-4
113 suppliers
$22.39/5ML
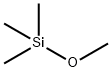
1825-61-2
239 suppliers
$15.00/25g

108-94-1
539 suppliers
$12.00/50g

933-40-4
113 suppliers
$22.39/5ML

108-94-1
539 suppliers
$12.00/50g

149-73-5
525 suppliers
$12.00/5g

933-40-4
113 suppliers
$22.39/5ML
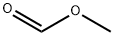
107-31-3
337 suppliers
$16.00/25mL