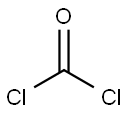
1,1-Dichlorodimethyl ether synthesis
- Product Name:1,1-Dichlorodimethyl ether
- CAS Number:4885-02-3
- Molecular formula:C2H4Cl2O
- Molecular Weight:114.96

79-37-8
475 suppliers
$17.67/10gm:
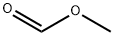
107-31-3
345 suppliers
$16.00/25mL

4885-02-3
188 suppliers
$10.00/1g
Yield:4885-02-3 71%
Reaction Conditions:
with N-methyl-N-phenylformamide at 25 - 30; for 31 h;
Steps:
Method A
Oxalyl chloride 127?g (1?mol) was slowly added dropwise to a mixture of methyl formate 90?g (1.5?mol) and N-methylformanilide 13.5?g (0.1?mol) at 25-30?°C for 7?h. (the condenser was cooled with ice-water) The mixture was stirred for a further 24?h at the same temperature until generation of the gas had stopped. The mixture was distilled through a 300?mm vigreux column to give 81.4?g of 2a (71% yield) having bp 83-84?°C (lit 6a 82-85.5?°C). GCMS: m/z?=?79 (M-35, base peak): 81 (M-35+2)?=?3:1. 1H NMR (300?MHz, CDCl3): δ 3.68 (s, 3H, CH3), 7.34 (s, 1H, CH). 13C NMR (75?MHz, CDCl3): δ 52.56, 98.82.
References:
Kimura, Yoshikazu;Warashina, Takuya [Tetrahedron Letters,2017,vol. 58,# 49,p. 4598 - 4599]
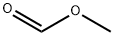
107-31-3
345 suppliers
$16.00/25mL

4885-02-3
188 suppliers
$10.00/1g