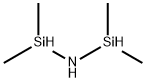
1,1,3,3-Tetramethyldisilazane synthesis
- Product Name:1,1,3,3-Tetramethyldisilazane
- CAS Number:15933-59-2
- Molecular formula:C4H15NSi2
- Molecular Weight:133.34
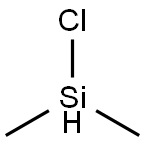
1066-35-9
233 suppliers
$28.00/25mL

15933-59-2
164 suppliers
$15.00/5 g
Yield:15933-59-2 197 g
Reaction Conditions:
with ammonia in pentane at -25 - 20; for 6 h;Schlenk technique;Inert atmosphere;
Steps:
1
[Example lj Synthesis of dimethylaminodimethylsilyl bisdimethylsilyl amine400g (4.23mo1) of chlorodimethylsilane((CH3)2HSiC1) and 1500m1 of n-pentane(organic solvent) were put into in a 3000mL flame-dried Schlenk flask under anhydrous and inert atmosphere while stirring, and 216g (12.68mo1) of ammonia (NH3 ) was slowly added thereto while maintaining a temperature at -25°C. After the addition was completed, the reaction solution was slowly heated to room temperature and stirred for 6 hours. The produced white solid was removed by filtering the reaction mixture after reaction was terminated, thereby obtaining a filtrate. Then, a solvent was removed from this filtrate under reduced pressure, thereby recovering 197g (1 .48mo1) of tetramethyldisilazane (((CH3)2HSi)2NH). 197g (1.48mo1) of the recovered tetramethyldisilazane (((CH3)2HSi)2NH) and 300m1 of n-hexane (organic solvent) were put into a 2000mL flame-dried Schlenk flask while stirring, and 456g (1.48mo1) of 2.29M normal butyl lithium (n-C4H9Li) hexane (C6H14) solution was slowly added thereto while maintaining a temperature at -15°C. After the addition was completed, the reaction solution was slowly heated to room temperature and stirred for 12 hours. Then, 500m1 of tetrahydrofuran (O(C2H2)2) was added thereto. 203g (1.48mo1) of chlorodimethyl dimethylaminosilane (ClSi(CH3)2(N(CH3)2)) synthesized by reacting dichloro dimethylsilane (C125i(CH3)2) with 2 equivalents of dimethylamine in a quantitative scheme was slowly added to the reaction solution while maintaining a temperature at -20°C. After the addition, the reaction solution was slowly heated, and stirred for 12 hours while maintaining the temperature at 65°C. The produced white solid was removed by filtering the reaction mixture after the reaction was terminated, thereby obtaining a filtrate. A solvent was removed from this filtrate under reduced pressure, and 208g (0.89mo1) of dimethylaminodimethylsilyl bisdimethylsilyl amine (((CH3)2SiH)2N(Si(CH3)2(N(CH3)2)) was obtained with a yield of 60% through distillation under reduced pressure.[851 A vapor pressure of dimethylaminodimethylsilyl bisdimethylsilyl amine (((CH3)2 SiH)2N(Si(CH3)2(N(CH3)2)) was measured and thermogravimetric analysis thereof was performed, and the results were illustrated in FIGS. 1 and 2.[861 1H-NMR(inC6D6) ? 0.20(s, 6H, NSi(CH3)2N(CH3)2), 0.23(d, 12H, NSiH(CH3)2), 2.46(s, 6H, Si(N(CH3)2), 4.68(m, 2H, NSiH(CH3)2);[871 Boiling point: 197°C;[881 GC analysis result> 99%.
References:
DNF CO., LTD.;JANG, Se Jin;LEE, Sang-Do;KIM, Jong Hyun;KIM, Sung Gi;JEON, Sang Yong;YANG, Byeong-il;SEOK, Jang Hyeon;LEE, Sang Ick;KIM, Myong Woon WO2015/190749, 2015, A1 Location in patent:Paragraph 83; 84; 85; 86; 87; 88

1066-35-9
233 suppliers
$28.00/25mL
999-97-3
640 suppliers
$9.00/5 g
75-77-4
568 suppliers
$5.04/10

15933-59-2
164 suppliers
$15.00/5 g