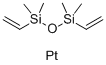The Role and Synthesis of Platinum(0)-1,3-Divinyl-1,1,3,3-Tetramethyldisiloxane Complexes in Catalysis
Mar 6,2024
General description
Catalysis represents a foundational pillar in the edifice of modern synthetic chemistry, acting as a key facilitator for the efficient and selective synthesis of complex molecules. Among the plethora of catalysts employed, platinum-based catalysts, particularly those in the zero oxidation state, stand out for their unparalleled efficacy in driving a wide array of chemical transformations. These range from the hydrogenation of unsaturated bonds to the hydrosilylation of alkynes and alkenes. Within this cadre, the Platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex emerges as a notable figure, heralding a new era in catalysis with its exceptional stability, reactivity, and selectivity[1].

Fig. 1 Traits of Platinum (0) -1,3-divinyl-1,1,3,3-tetramethyldisiloxane
Synthesis
The synthesis of these distinguished Platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complexes is typically achieved through the reaction between a platinum precursor, such as Pt(PPh_3)_4, and 1,3-divinyl-1,1,3,3-tetramethyldisiloxane. This reaction, meticulously conducted under stringently controlled conditions, yields a complex in which the platinum atom is adeptly coordinated to the vinyl groups of the disiloxane, resulting in a zero-valent platinum center of remarkable stability. The synthesis's success is intricately linked to the judicious selection of reaction parameters, including the choice of solvent, temperature, and platinum source, each of which plays a critical role in dictating the yield and purity of the resultant complex[2].
Properties and Reactivity
The synthesized Platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex is distinguished by its unique set of properties, which render it an exemplary catalyst. Its robust stability across diverse reaction conditions is particularly laudable, enabling its deployment in a spectrum of reactions prone to decomposition or undesirable side reactions. Moreover, the complex's proficiency in activating both C-H and Si-H bonds opens the door to a plethora of efficient and selective organic transformations, underscoring its versatility and utility in organic synthesis.
Catalytic Applications
The catalytic prowess of the Platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex is manifested in its wide-ranging applications, most notably in facilitating cross-coupling reactions that are crucial for the formation of carbon-carbon bonds. Additionally, its efficacy in the hydrosilylation of alkenes and alkynes is of significant industrial and academic interest, offering a sustainable pathway to silicon-containing materials with varied applications, from the fabrication of medical devices to the development of advanced coatings. The complex's utility is further extended to cyclization and polymerization reactions, highlighting its broad applicability and indispensability in the realm of synthetic chemistry.
Challenges and Future Directions
Despite the formidable catalytic capabilities of the Platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex, certain challenges persist in its application. Its sensitivity to moisture and air poses practical challenges, necessitating stringent handling and storage protocols to preserve its activity. Moreover, the ongoing quest for more sustainable and environmentally friendly catalytic processes remains a pivotal driving force in the field of catalysis. Future research endeavors may pivot towards the modification of the ligand structure to bolster the complex's stability and reactivity or the exploration of its utility in novel catalytic processes. These pursuits hold the promise of unlocking new avenues in catalysis, further enriching our capacity to synthesize complex molecules and materials with precision and efficiency.
Storage Methods
The proper storage of the Platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex is paramount to preserve its catalytic activity and stability, given its sensitivity to moisture and air. To mitigate these vulnerabilities, the complex should be stored under an inert atmosphere, such as argon or nitrogen, in airtight containers. This precaution prevents oxidative degradation and moisture-induced decomposition, ensuring the complex remains in its active form for catalytic applications.
For long-term storage, refrigeration at temperatures below 0°C, typically between -20°C to -40°C, is recommended. This low-temperature environment reduces the rate of any potential decomposition reactions that could compromise the complex's integrity over time. However, it is crucial to allow the container to reach room temperature under an inert atmosphere before opening to avoid condensation of atmospheric moisture on the cold complex.
Additionally, it is advisable to store the complex in small aliquots to minimize exposure to air and moisture each time the storage container is accessed. This practice extends the shelf life of the complex by reducing the frequency and duration of exposure to potentially degrading conditions.
Employing these storage methods will significantly enhance the longevity and performance of the Platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex, ensuring its readiness for use in catalytic processes without necessitating additional purification steps prior to use[3].
References
[1]Pesti, Jaan, and Gerald L. Larson. "Tetramethyldisiloxane: a practical organosilane reducing agent."Organic Process Research & Development20.7 (2016): 1164-1181.
[2] Lucas, Glennard R., and Robert W. Martin. "Tetramethyldisiloxane-1, 3-diol."Journal of the American Chemical Society74.20 (1952): 5225-5225.
[3]Iimura, Tomohiro, et al. "A Pt (0) complex with cyclic (alkyl)(amino) silylene and 1, 3-divinyl-1, 1, 3, 3-tetramethyldisiloxane ligands: synthesis, molecular structure, and catalytic hydrosilylation activity."Dalton Transactions46.27 (2017): 8868-8874.
- Related articles
- Related Qustion
- Platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane: applications and safety Nov 13, 2023
Platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane is a versatile catalyst for hydrosilylation, widely used in various industries. Caution should be exercised due to its flammability.
- Applications of Karstedt Catalysts Dec 21, 2021
Karstedt catalysts promote a quick, low temperature curing of the hydrosilylation reaction. They form silicone elastomers through an addition curing cross-linking reaction between Si-vinyl and Si-H groups.
Perfluorotributylamine, valued for stability in electronics and tumor treatment, poses environmental and health risks requiring regulatory control and alternative exploration.....
Nov 13,2024APIFmoc-Phe-OH is a key compound in the field of peptide synthesis, playing an important role as a building block in the process of peptide assembly.....
Mar 6,2024APIYou may like
Platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane manufacturers
- Platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane
-

- $10.00 / 1kg
- 2024-11-15
- CAS:68478-92-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- Platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane;Pt catalyst
-

- $1.00 / 1Kg/Drum
- 2024-11-07
- CAS:68478-92-2
- Min. Order: 0.1KG
- Purity: Pt content: 0.1-10%
- Supply Ability: 1mt per month
- Platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane Platinum
-

- $1.00 / 1KG
- 2024-11-04
- CAS:68478-92-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10mt