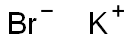The preparation method of potassium bromide
Apr 8,2022
Introduction
Potassium bromide is an inorganic substance with a chemical formula of KBr and a relative molecular mass of 119[1]. Colorless crystal or white powder, with strong salty taste, turns yellow when exposed to light. Slightly hygroscopic. 1g is dissolved in 1.5ml of water, and the aqueous solution is neutral. The relative density was 2.75 (25°C). Melting point 730 ℃. Boiling point 1435 ℃. irritating. Mainly used for spectroscopic analysis, spot analysis of copper and silver, polarographic analysis of indium, cadmium and arsenic, and developer.

Picture 1 Potassium bromide powders
Potassium bromide is a colorless cubic crystal. Odorless, salty and slightly bitter. It turns yellow easily when exposed to light and is slightly hygroscopic. Soluble in water (102g/100ml water at 100°C) and glycerol, slightly soluble in ethanol and ether. The aqueous solution is neutral. Its bromide ion can be replaced by fluorine and chlorine. Reacts with sulfuric acid to generate hydrogen bromide. It reacts with silver nitrate to form a yellow silver bromide precipitate.
The preparation method of potassium bromide
Electrolysis. The potassium bromide synthesized by bromine and potassium hydroxide is dissolved in distilled water to form an electrolyte, and the first batch of crude products is obtained after electrolysis for 24 hours[2]. After that, crude products are taken every 12 hours. The crude products are washed with water to remove potassium bromide and then hydrolyzed by distillation. The pH value is adjusted to 8 with potassium hydroxide, and the product is filtered after being kept for 0.5h.
Chlorine oxidation. After the lime milk and the bromine are reacted, chlorine gas is introduced to carry out the chlorine oxidation reaction, and the reaction is terminated when the pH value reaches 6~7. After deslagging, the filtrate was evaporated. Add barium chloride solution to react to generate barium bromate precipitation, add water to suspend the filtered precipitation to maintain a certain temperature and add potassium carbonate to carry out metathesis reaction, the crude potassium bromate is washed with a small amount of distilled water for many times, filtered, evaporated, crystallized by cooling, separated, dried, Pulverized to obtain edible potassium bromate product.
Bromine-potassium hydroxide method. Take industrial bromine and potassium hydroxide as raw materials, dissolve potassium hydroxide with 1.4 times the mass of water to make a solution, and feed bromine under constant stirring. When the bromine is added to a certain amount, white crystals will be precipitated to obtain the crude potassium bromate.
Continue adding bromine until the liquid is pink. While adding bromine, continuously add cold water to the solution to prevent bromine volatilization loss due to high temperature. Repeated recrystallization, filtration, drying, and then dissolved in deionized water, adding a small amount of potassium hydroxide to remove excess bromine during synthesis, recrystallization once, and finally taking out the crystals and drying to obtain the finished product.
Storage and use of potassium bromide
Avoid ingestion or inhalation, avoid eye and skin contact. If ingested, dizziness and nausea may occur, seek medical attention immediately; if inhaled, vomiting may occur, immediately move the patient to fresh air and seek medical attention; if splashed into the eyes, immediately use a large amount of fresh Rinse with water for 20min; if the skin comes into contact with potassium bromide, also rinse with plenty of water. Medicines should be sealed and stored in a dry place away from light. Packed in two-layer paper bags lined with polyethylene plastic bags and outer corrugated boxes, each with a net weight of 20kg, 25kg or 50kg. It should be stored in a ventilated and dry warehouse. The packaging should be complete, pay attention to moisture-proof and light-proof. Protect from rain and sunlight when transporting. When loading and unloading, handle with care to prevent the package from being damaged. In case of fire, sand and various fire extinguishers can be used to put out the fire.
Reference
1 Ma Wanshan, Zhong Li, Gao Suying, Li Ming. Extraction and separation of gold by ammonium sulfate-potassium bromide-ethanol system. 2004
2 Duan Hui, Liu Zhongfang, Hu Xiaoli, Liu Shaopu. KIO3-Potassium Bromide-Methyl Violet System Resonance Rayleigh Scattering Determination of Penicillin Antibiotics. 2008
- Related articles
- Related Qustion
- What are the use and side effects of Potassium bromide Sep 4, 2024
Potassium bromide may permit humans to decrease the dose level of the phenobarbital. Potassium bromide may also be used initially as the sole anticonvulsant drug.
Potassium bromide
7758-02-3You may like
Potassium bromide manufacturers
- Potassium bromide
-

- $6.00 / 1kg
- 2024-11-18
- CAS:7758-02-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000KG/Month
- potassium bromide
-

- $85.00 / 1kg
- 2024-11-18
- CAS:7758-02-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- Potassium bromide
-

- $6.00 / 1kg
- 2024-11-18
- CAS:7758-02-3
- Min. Order: 1kg
- Purity: More than 99%
- Supply Ability: 2000KG/Month