Synthesis of phthalide and its biological information
Aug 25,2022
General description
Phthalide is a natural product found in Ligusticum sinense and Ligusticum chuanxiong with data available. Phthalides are a relatively small group of natural compounds confined to several plant families and some genera of fungi and liverworts[1-2]. They are divided into two structural groups, the monomeric and dimeric phthalides, and known mainly as bioactive constituents of different plant species used traditionally for medicinal purposes in Asia, Europe, and North America[3].
Different chromatographic, spectrometric, and two-dimensional nuclear magnetic resonance (NMR) techniques have been used for the isolation and structural characterization of phthalides in extracts, and for assessing the quality of plant material containing this type of compound. Isotopic labeling has established the biosynthesis of phthalides via linkage of acetate units forming polyketide intermediates[4].
Chemical transformations of monomeric phthalides have included oxidation, reduction, addition, elimination, and cycloaddition reactions, and treatments with Lewis acids of (Z)-ligustilide have afforded linear dimers[5-6]. Some intramolecular condensations and differentiated cyclizations of the dimeric phthalides have been carried out, providing evidences for the particular chemical reactivity of these compounds.
Several structural modifications of phthalides have been carried out subjecting them to microbial transformations by different species of bacteria, fungi and algae, and these included resolutions of racemic mixtures and oxidations, among others[7].
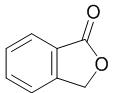
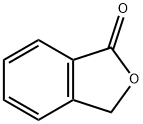
Fig. 1 The structure of phthalide.
Synthesis
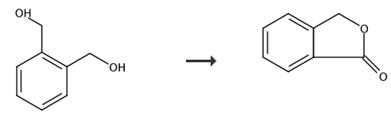
Fig. 2 The synthetic route of phthalide[8].
To a 100 mL round-bottom flask were added Fe(NO3)3·9H2O (40.5 mg, 0.1 mmol) and DCE (3.0 mL). Then TEMPO (15.6 mg, 0.1 mmol), KCl (7.5 mg, 0.1 mmol), 1a (183.8 mg, 1.0 mmol), and DCE (1.0 mL) were added sequentially with stirring. The flask was connected to a gas bag of air with a valve. The reaction was then carried out at room temperature until completion of the reaction as monitored by TLC (petroleum ether/ethyl acetate = 5/1) (12 h). The crude reaction mixture was filtered through a pad of silica gel eluted with ether (75 mL). After evaporation, the residue was purified by chromatography on silica gel. 2a (176.5 mg, 88%) (eluent: petroleum ether/ethyl acetate = 5/1) as a pale yellow solid:1 (3) Octanoic acid (2c) (zjs-6-145) The reaction of Fe(NO 3)3·9H2O (40.5 mg, 0.1mmol), TEMPO (15.7 mg, 0.1 mmol), KCl (7.5 mg, 0.1 mmol), 1c (128.7mg, 1.0 mmol), and DCE (4.0 mL). (18) 1(3H)-Isobenzofuranone (4v) (jxg-3-54) The reaction of 3v (141.3 mg, 98% purity, 1.0 mmol), Fe(NO3)3·9H2O (40.6 mg, 0.1 mmol), TEMPO (15.5 mg, 0.1 mmol), and KCl (7.7 mg, 0.1 mmol) in DCE (4 mL) for 16 h. 4v (88.3 mg, 66%) (eluent: petroleum ether/ethyl acetate = 15/1 to 10/1) as a solid:35 1H NMR (400 MHz, CDCl3) δ 7.92 (d, J = 7.6 Hz , 1 H, Ar-H), 7.70 (td, J1 = 7.6 Hz, J2 = 1.2 Hz, 1 H, Ar-H), 7.58-7.49 (m, 2 H, Ar-H), 5.34 (s, 2 H, CH2); 13C NMR (100 MHz, CDCl3) δ 171.1, 146.5, 134.0, 129.0, 125.62, 125.57, 122.1, 69.6.
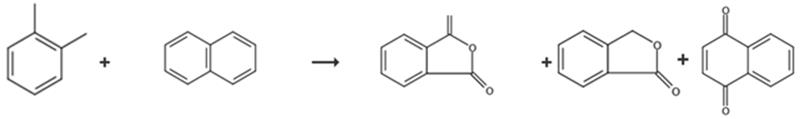
Fig. 3 The synthetic route of phthalide[9].
The tube was flows through every hour from top to bottom with 4.0 Nm3 air having loadings of 99 to 99.4 wt.-% o-xylene from 0 to 40 g/Nm3 and technical naphthalene of 37-42 g/Nm3. After days run time, an air flow of 4 Nm3/h, an o-xylene loading of 10 g/Nm3, and a naphthalene loading of 41.2 g/Nm3 was interrupted the supply of the reactant gas to the catalyst for 2 hours at a salt bath temperature of 377°C and replaced with nitrogen. After the 2 hours, the catalyst was again applied at conditions identical to those loaded prior to the shutdown with o-xylene and naphthalene laded air, i.e. a salt bath temperature of 377°C, an air flow of 4 Nm3/h, an o-xylene loading of 10 g/Nm3 and a naphthalene loading of 41.2 g/Nm3. After another day the product gas composition was analyzed. Phthalic anhydride, phthalide, naphthoquinone.
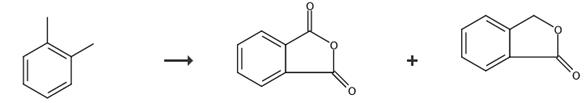
Fig. 4 The synthetic route of phthalide[10].
The tube was flows through by every hour from top to bottom with 4.0 Nm3 air having loadings of 99 to 99.4 wt .-% sodium o-xylene of 30 to 100 g/Nm3. After 172 days, maturity was at a salt bath temperature of 346°C, an air flow of 4 Nm3/h and an o-xylene loading of 100 g/Nm3 interrupted the supply of reactant gas to the catalyst for 6 hours and replaced with nitrogen (reactor inlet pressure = 260 mbar). After the 6 hours, the catalyst was again applied at conditions identical to those preceding the detachment with o-xylene-laden air, ie a salt bath temperature of 346 °C, an air flow of 4 Nm3/h and an o-xylene loading of 100 g/Nm3. After four more days, the product gas composition was analyzed. Products: phthalic anhydride, Phthalide.
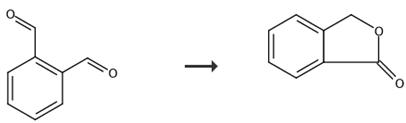
Fig. 5 The synthetic route of phthalide[11].
In a glove box, to a reaction vessel equipped with a stirring bar was placed [(η3-PhCOCF2)Ni(dcpe)][FB(C6F5)3] (11.7 mg, 0.01 mmol). The solid was dissolved in 0.5 mL of toluene. To the solution was added an aldehyde (1.0 mmol). The reaction mixture was stirred at ambient temperature for 1 h. 8a 5 8m 12. The product was obtained by following the general procedure (2 mol% of nickel catalyst was used) and purified by kugelrohr distillation to give white solid (51.0 mg, 76%). 1H NMR (400 MHz, in CDCl3, rt, δ/ppm): 5.35 (s, 2H), 7.55 (m, 2H), 7.71 (dt, J = 1 Hz, 7.5 Hz, 1H), 7.92 (d, 7.6 Hz, 1H). 13C NMR (100 MHz, in CDCl3, rt, δ/ppm): 69.7, 122.2, 125.7, 129.1, 134.1, 146.6, 171.1.
Anticonvulsive Effects
Epilepsy is a disorder characterized by abnormal neuronal electrical activity with periodic and unpredictable seizures[12]. Phthalide was shown to be anticonvulsive agents against metrazole, electroshock-, and audio-induced seizures. In the experiment, the anticonvulsive effects of both phthalides. rac-Butylphthalide protected against chronic epilepsy induced by coriaria lactone, and at 700 mg/kg prevented abnormalities in the hippocampus[13].
Progestogenic Effects
The hormone progesterone is necessary for menstrual and reproductive health. During menopause, hormone replacement therapy is an effective treatment against hormonal disorders. Phthalides have shown progesterone-like activity[14]. For example, phthalide (EC50?=?91 nM) was shown to be a potent and specific activator of the progesterone receptor, with riligustilide (24) (EC50?=?81 μM) displaying weaker activity. Levistolide A ((23) (Z,Z′)-diligustilide) was inactive, which demonstrates the importance of minor structural variations in this type of molecule for biological activity.
Cytotoxic Effects
The current lack of specificity for multiple antitumor therapies has led to a search for novel, more targeted agents. Phthalide is an agent that has been tested for activity against the serine/threonine-protein kinase Akt1, which regulates metabolism, proliferation, and cell survival, and showed an IC50 value of 19.7 μM[15]. The IC50 for the functional inhibition of Bad phosphorylation by Akt1 was 30.4 μM.
Bioavailability and Routes of Administration
The absorption, distribution, metabolism and excretion of phthalide after hot or cold dermal administration have been evaluated. Phthalide was subsequently detected in the liver, bile, and kidney at 0 h, and in the intestinal contents at 4 h[16]. Radioactivity was maximal at 0 h in the skin and plasma (and then decreased, t1/2 0.5–1 h), and was sustained in the liver, bile, and kidney until 1 h, and thereafter accumulated in the small and large intestines, cecum and its contents, reaching maximal values 1–2 h later. Altogether, 70% of the unaltered or metabolized phthalide was captured from the urine at 8 h, increasing to 80% within 24 h; only 5% was excreted into the feces within 24 h[16-18]. The cysteine conjugate was detected in both the urine and feces. It was demonstrated that phthalide immediately permeated through the skin into the circulatory system[19].
Reference
[1] A.R. Ayupova, T.A. Yangirov, R.K. Yumagulova, A.A. Fatykhov, V.A. Kraikin, Effect of Synthesis Conditions on the Regularities of Formation of Arylenephthalide-Styrene Copolymers, Russ. J. Gen. Chem. 92(6) (2022) 996-1004.
[2] P. Basu, N. Satam, S. Pati, A. Suresh, I.N.N. Namboothiri, Reactions of Sulfonylphthalide with Diverse Activated Imines for the Synthesis of Enaminophthalides, Spiro-isoquinolinones, and Homalicine Natural Products, J. Org. Chem. (2022) Ahead of Print.
[3] Y. Deng, Z. Cao, G. Yu, Q. Song, Z. Liu, S. Cong, Preparation of 3-(hydroxybenzyl) phthalide compounds for preparing pharmaceutical compositions, Sichuan University, Peop. Rep. China . 2022, p. 31pp.
[4] L. Fan, Y. Li, Z. Luo, L. Tang, Preparation of phthalide hydrazide compounds as fungicides, Guizhou Medical University, Peop. Rep. China . 2022, p. 22pp.
[5] Y. Hu, C. Hu, P. Yu, G. Yan, C. Li, H. Hu, Preparation of phthalide by hydrogenation of phthalic anhydride under atmospheric pressure, Dalian University of Technology, Peop. Rep. China; Jining Carbon Group Co., Ltd.; Shandong Chenyang New Carbon Material Co., Ltd. . 2022, p. 9pp.
[6] L. Huang, C. Peng, L. Guo, R. Feng, H.-Z. Shu, Y.-C. Tian, Q.-M. Zhou, L. Xiong, Six pairs of enantiomeric phthalide dimers from the rhizomes of Ligusticum chuanxiong and their absolute configurations and anti-inflammatory activities, Bioorg. Chem. 127 (2022) 105970.
[7] A. Kazantsev, O. Bakulina, D. Darin, G. Kantin, A. Bunev, M. Krasavin, Unexpected Ring Contraction of Homophthalic Anhydrides under Diazo Transfer Conditions, Org. Lett. 24(26) (2022) 4762-4765.
[8] P. Kumar, V. Rana, A.N. Singh, Angelica glauca Edgew. - A comprehensive review, J. Appl. Res. Med. Aromat. Plants 31 (2022) 100397.
[9] A. Li, Y. Song, L. Yu, R. Liu, A. Sun, Green method for extracting, separating and purifying phthalides from Ligusticum chuanxiong, Liaocheng University, Peop. Rep. China . 2022, p. 15pp.
[10] C. Liang, Q. Duan, C. Li, T. Li, A kind of phthalic anhydride hydrogenation of benzene phthalein crystallization mother liquor solute and solvent recovery system [Machine Translation], Chengdu Dayan Technology Industry Development Co., Ltd., Peop. Rep. China . 2022, p. 9pp.
[11] S.-Z. Lin, T.-L. Chiu, H.-J. Harn, T.-W. Chiou, J.-H. Lee, Use of phthalide compounds in treatment of meningioma, Everfront Biotech Inc., Peop. Rep. China . 2022, p. 43pp.
[12] N. Mansouri, O. Benslama, In vitro and in silico investigation of the antifungal activity of endophytic fungi against phytopathogenic fungi of tomato, Not. Sci. Biol. 14(1) (2022) 11050.
[13] C.A. Oliva, J. Stehberg, R. Barra, T. Mariqueo, Neuropathic Pain Induces Interleukin-1β Sensitive Bimodal Glycinergic Activity in the Central Amygdala, Int. J. Mol. Sci. 23(13) (2022) 7356.
[14] B. Ruan, X. Tang, W. Guo, Y. Hu, L. Chen, Synthesis and Biological Evaluation of Novel Phthalide Analogs-1,2,4-Oxadiazole Hybrids as Potential Anti-Inflammatory Agents, Chem. Biodiversity 19(8) (2022) e202200039.
[15] R.B. Salikhov, R.A. Zilberg, I.N. Mullagaliev, T.R. Salikhov, Y.B. Teres, Nanocomposite thin film structures based on polyarylenephthalide with SWCNT and graphene oxide fillers, Mendeleev Commun. 32(4) (2022) 520-522.
[16] X. Wei, Y. Zeng, C. Sun, F. Meng, Y. Wang, Recent advances in natural phthalides: Distribution, chemistry, and biological activities, Fitoterapia 160 (2022) 105223.
[17] S.-S. Yang, Y.-F. Chen, H.-H. Ko, H.-C. Wu, S.-Y. Hsieh, M.-D. Wu, M.-J. Cheng, H.-S. Chang, Undescribed alkyne-geranylcyclohexenetriols from the endophyte Diaporthe caulivora 09F0132 and their anti-melanogenic activity, Phytochemistry (Elsevier) 202 (2022) 113312.
[18] H. Zhou, J. Zhou, M. Huang, C. Hu, R. Chen, K. Zhang, G. Mei, C. Zhang, H. Xu, Y. Huang, J. Sun, Containing ligusticum chuanxiong, borneol, artificial musk compound combined moschus pharmaceutical formulation for characteristic spectrum construction method [Machine Translation], Sichuan New Green Pharmaceutical Technology Development Co., Ltd., Peop. Rep. China . 2022, p. 26pp.
[19] C. Zou, H. Zhang, Y. Zheng, C. Wu, 4 '-(hexaafluoroisopropenyl) dithalthanhydride anhydride and preparation method thereof [Machine Translation], Sanming HexaFluo Chemical Co., Ltd., Peop. Rep. China . 2022, p. 11pp.
- Related articles
- Related Qustion