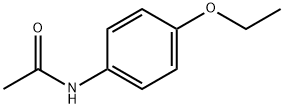
Phenacetin
Dec 10,2021
Phenacetin (p-Acetophenetidine), the chemical name is p-ethoxy-N-acetanilide, 4-ethoxy-N-acetanilide, p-acetanilide, and the chemical formula is C 10 H 13 NO 2, It is a white shiny scaly crystal or white crystalline powder at room temperature. It has a melting point of 137 ~ 138 ℃ and a boiling point of 132 ℃ /4 mmHg. It is hardly soluble in water and soluble in organic solvents such as ethanol, acetone, acetic acid, pyridine, and chloroform.
Pharmacodynamics
This product is an acetanilide antipyretic and analgesic, and its antipyretic and analgesic effect is mild and lasting. The antipyretic strength is similar to that of aspirin, but the analgesic effect is weaker. Few anti-inflammatory, anti-rheumatic and anti-platelet aggregation effects. Due to the greater toxicity and the replacement of paracetamol with similar effects and less toxicity, this product is no longer used alone. It is only used in compound preparations with aspirin, aminopyrine, and barbiturates.
Pharmacokinetics
The oral administration is rapid and complete. It reaches the peak blood concentration about 2 hours after oral administration. About 30% of the drug is bound to plasma proteins and quickly distributed in various tissues, but the concentration in the cerebrospinal fluid is very low. Mainly metabolized in the liver. Most (75%~80%) are deethylated in the liver to become therapeutic paracetamol; a small part is deacetylated to form toxic p-aminophene, which oxidizes hemoglobin to methemoglobin that loses its oxygen-carrying function , Resulting in symptoms such as cyanosis and hypoxia. ~ The duration of the administration is about 3-4h.
Toxic side effect
It has mild irritation to the gastrointestinal tract, rarely induces ulcers and bleeding, but often causes cyanosis, especially in children. Long-term application can cause hemolytic anemia, damage the liver and kidneys, and even renal papillary necrosis, uremia, etc. Occasionally, irritability, listlessness, sweating, vomiting, convulsions, autocytopenia, skin rash, exfoliative dermatitis, etc. are common.
Mechanism of action
It is easily absorbed by oral administration, and reaches the highest concentration in the blood within 1 to 2 hours, and is distributed in various tissues, but the concentration in the cerebrospinal fluid is very low. Approximately 80% is deethylated in the liver to become acetaminophen, so the concentration of acetaminophen in the blood rises faster. The other part is deacetylated into p-aminophenethyl ether. Para-aminophenethyl ether and a small part of paracetamol are further changed into para-aminophenol, which are toxic substances that cause hemoglobin denaturation, hemolysis and liver damage.
Effect
(1) It is an aniline antipyretic and analgesic drug. Acetaminophen is a metabolite of phenacetin in the body. Its antipyretic and analgesic effect is similar to that of aspirin.
(2) The anti-inflammatory and anti-rheumatic effects are very weak, have no curative effect, have no irritation to the gastrointestinal tract, and will not cause coagulation disorders.
(3) It has a similar antidiuretic effect, can promote water reabsorption, and has a certain effect on diabetes insipidus.
- Related articles
- Related Qustion
- Phenacetin: A Comprehensive Overview for Chemistry Professionals Oct 31, 2024
Phenacetin, a painkiller, was the world's first synthetic pharmaceutical drug. It was one of the first painkillers that was not derived from opium while at the same time being absent of anti-inflammatory qualities.
- Phenacetin: pharmacokinetics, mechanism of action and clinical applications Aug 23, 2023
Phenacetin is absorbed orally, metabolized to paracetamol and inhibits prostaglandin synthesis. no longer widely used due to safety concerns.
- The Side Effects of Phenacetin Jul 4, 2023
henacetin is an analgesic and antipyretic drug that was commonly used in the early 20th century to relieve pain and reduce fever.
Caffeic acid is an organic compound that is classified as a hydroxycinnamic acid. This yellow solid consists of both phenolic and acrylic functional groups. It is found in all plants because it is a key intermediate in the biosynthesis of lignin, one of the principal components of woody plant biomass and its residues.....
Aug 27,2019APIDimethyl acetylenedicarboxylate (DMAD) is an electron-deficient acetylenic compound having two reactive ester groups. It is a privileged and advantaged molecule, which participates easily and practically in heterocyclization. Due to the presence of two ester electron-withdrawing group, DMAD easily undergoes Michael addition, which is followed by heterocyclization to afford versatile heterocyclic compounds with different ring sizes. DMAD carries out Diels–Alder reactions under mild conditions to give heterocycles, which could not be easily synthesized via conventional heterocyclizations.....
Aug 27,2019Organic reagentsPhenacetin
62-44-2You may like
- Phenacetin
-
- $45.00 / 500mg
- 2024-11-16
- CAS:62-44-2
- Min. Order:
- Purity: 99.86%
- Supply Ability: 10g
- Phenacetin
-
- $45.00 / 500mg
- 2024-11-16
- CAS:62-44-2
- Min. Order:
- Purity: 99.86%
- Supply Ability: 10g
- Phenacetin
-

- $30.00 / 1kg
- 2024-11-16
- CAS:62-44-2
- Min. Order: 1kg
- Purity: 99.9%
- Supply Ability: 10000