GMP GENIUS?Nuclease
優(yōu)勢特色(Features)
Designed under ISO 9001:2015 and ISO 13485:2016;
Manufactured and QC tested under a GMP compliance factory;
FDA DMF filed;
Animal-Free materials;
Batch-to-batch consistency;
Stringent quality control tests;
No animal derived peptone and lactose used in production process.
來源(Source)
GMP GENIUS?Nuclease is a tag free recombinant form of Serratia marcescens extracellular endonuclease produced in Escherichia coli cells using a proprietary process at ACRObiosystems. GMP GENIUSNuclease is a homodimer with monomer molecular masses about 30 kDa. The enzyme is a non-specific nuclease with high specific activity, which degrades both single- and double-stranded nucleic acids in any form (single stranded, double stranded, linear, circular and supercoiled). It hydrolyzes internal phosphodiester bonds present between the nucleotides to 5’- phosphorylated oligonucleotides of 3-5 bases in length.
Predicted N-terminus: Met.
應用說明(Application)
Elimination of nucleic acids from biologics
clinical viral vaccine production;
clinical viral vector production for cell and gene therapy(CGT);
Other clinical development and production uses.
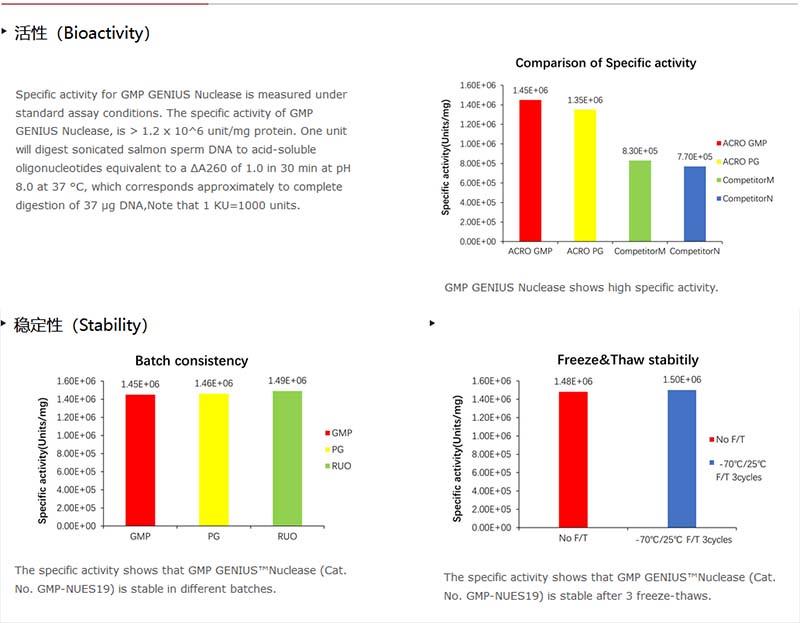
酶活(Enzyme Activity)
≥250 U/μL
宿主蛋白殘留(Host Cell Protein)
<0.05ng/ug of protein tested by ELISA.
反應條件(Operating conditions)
GMP GENIUS?Nuclease is functional between pH 6 and 10 (optimal at pH8 - 8.5) , and from 0°C to 50 °C (optimal at 37 °C - 45 °C). Mg2+ (1-2 mM) is required for enzyme activity.1 mM EDTA reduced the activity by 30% in the presence of 1 mM MgCl2; 0.1 M EDTA eliminated all enzyme activity. In the presence of 1 mM MgCl2, enzyme levels were reduced 75% by 0.1 M CaCl2 or 1 M NaCl. Under standard assay conditions, 1 mM iodoacetate had no effect on the enzymatic rate, whereas 1 mM mercaptoethanol and maleic acid reduced the activity by only 5 to 10%. 10 mM p- Chloromercurybenzoate completely inactivates the enzyme, while 0.64 M beta-mercaptoethanol in the presence of 2 M urea causes only partial inactivation of the enzyme. 4 or 7 M Urea increases the enzyme activity.
內(nèi)毒素(Endotoxin)
Less than 0.01 EU per μg by the LAL method.
蛋白酶活性(Protease activity)
Negative
無菌(Sterility)
The sterility testing was performed by membrane filtration method.
支原體(Mycoplasma)
Negative.
重金屬殘留(Heavy Metals)
≤10ppm
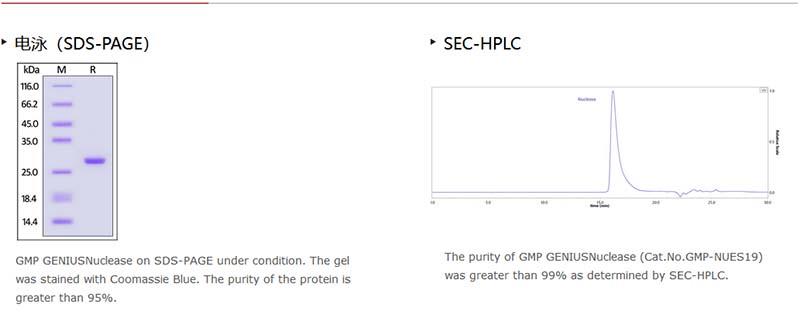
純度(Purity)
≥95% as determined by SDS-PAGE.
≥99% as determined by SEC-HPLC.
制劑(Formulation)
Supplied as 0.2 μm filtered solution in 20 mM Tris, 20 mM NaCl, 2 mM MgCl2, pH8.0 with glycerol as protectant.
Contact us for customized product form or formulation.
運輸(Shipping)
This product is supplied and shipped with dry ice, please inquire the shipping cost.
存儲(Storage)
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
The product MUST be stored at -20°C or lower upon receipt;
-20°C for 5 years under sterile conditions.
MANUFACTURING SPECIFICATIONS
ACROBiosystems GMP grade products are produced under a quality management system and in compliance with relevant guidelines: Ph. Eur General Chapter 5.2.12 Raw materials of biological origin for the production of cell-based and gene therapy medicinal products; USP <92> Growth Factors and Cytokines Used in Cell Therapy Manufacturing; USP <1043> Ancillary Materials for Cell, Gene, and Tissue-Engineered Products; ISO/TS 20399-1:2018, Biotechnology - Ancillary Materials Present During the Production of Cellular Therapeutic Products.
ACROBiosystems Quality Management System Contents:
Designed under ISO 9001:2015 and ISO 13485:2016, Manufactured and QC tested under a GMP compliance factory.
Animal-Free materials;
Materials purchased from the approved suppliers by QA;
ISO 5 clean rooms and automatic filling equipment;
Qualified personnel;
Quality-related documents review and approve by QA;
Fully batch production and control records;
Equipment maintenance and calibration;
Validation of analytical procedures;
Stability studies conducted;
Comprehensive regulatory support files
ACROBiosystems provide rigorous quality control tests (fully validated equipment, processes and test methods) on our GMP grade products to ensure that they meet stringent standards in terms of purity, safety, activity and inter-batch stability, and each bulk QC lot mainly contains the following specific information:
SDS-PAGE;
Protein content;
Endotoxin level;
Residual Host Cell DNA content;
Residual Host Cell Protein content;
Biological activity analysis;
Microbial testing;
Mycoplasma testing;
In vitro virus assay;
Batch-to-batch consistency
DISCLAIMER
ACROBiosystems GMP grade products are designed for research, manufacturing use or ex vivo use. CAUTION: Not intended for direct human use.
TERMS AND CONDITIONS
All products are warranted to meet ACROBiosystems Inc.’s (“ACRO”) published specifications when used under normal laboratory conditions.
ACRO DOES NOT MAKE ANY OTHER WARRANTY OR REPRESENTATION WHATSOEVER, WHETHER EXPRESS OR IMPLIED, WITH RESPECT TO ITS PRODUCTS. IN PARTICULAR, ACRO DOES NOT MAKE ANY WARRANTY OF SUITABILITY, NONINFRINGEMENT, MERCHANTABILITY OR FITNESS FOR ANY PARTICULAR PURPOSE.
NOT WITH STANDING ANY OTHER PROVISIONS OF THESE TERMS AND/OR ANY OTHER AGREEMENT BETWEEN ACRO AND PURCHASER FOR THE PURCAHSE OF THE PRODUCTS, ACRO’S TOTAL LIABILITY TO PURCHASER ARISING FROM OR IN RELATION TO THESE TERMS, AN AGREEMENT BETWEEN THE PARTIES OR THE PRODUCTS, WHETHER ARISING IN CONTRACT, TORT OR OTHERWISE SHALL BE LIMITED TO THE TOTAL AMOUNT PAID BY PURCHASER TO ACRO FOR THE RELEVANT PRODUCTS. IN NO EVENT WILL ACRO BE LIABLE FOR THE COST OF PROCUREMENT OF SUBSTITUTE GOODS.
END USER TERMS OF USE OF PRODUCT
The following terms are offered to you upon your acceptance of these End User Terms of Use of Product. By using this product, you indicate your acknowledgment and agreement to these End User Terms of Use of Product. If you do not agree to be bound by and comply with all of the provisions of these End User Terms of Use of Product, you should contact your supplier of the product and make arrangements to return the product.
The End User is aware that ACROBiosystems Inc. and its affiliate (“ACRO”) sell GMP grade products designed for research, manufacturing use or ex vivo use and not intended for human in vivo applications. The End User further agrees, as a condition of the sales of ACRO’s GMP grade products that: a) the End User will not use this GMP grade product in any procedure wherein the product may be directly or indirectly administered to humans, unless the End User has obtained, or prior to their use will have obtained, an Investigational New Drug (IND) exemption from the FDA and will use the product only in accordance with the protocols of such IND and of the Institutional Review Board overseeing the proposed research, or b) the End User will use the products outside of the United States in accordance with the protocols of research approved by the applicable review board or authorized ethics committee and regulatory agencies to which the End User is subject to in their territory.
背景(Background)
CRISPR (clustered regularly interspaced short palindromic repeat) is an adaptive immune system that provides protection against mobile genetic elements (viruses, transposable elements and conjugative plasmids),CRISPR clusters contain spacers, sequences complementary to antecedent mobile elements, and target invading nucleic acids. CRISPR clusters are transcribed and processed into CRISPR RNA (crRNA). In type II CRISPR systems correct processing of pre-crRNA requires a trans-encoded small RNA (tracrRNA), endogenous ribonuclease 3 (rnc) and this protein. The tracrRNA serves as a guide for ribonuclease 3-aided processing of pre-crRNA; Cas9 only stabilizes the pre-crRNA:tracrRNA interaction and has no catalytic function in RNA processing. Subsequently Cas9/crRNA/tracrRNA endonucleolytically cleaves linear or circular dsDNA target complementary to the spacer; Cas9 is inactive in the absence of the 2 guide RNAs (gRNA). The target strand not complementary to crRNA is first cut endonucleolytically, then trimmed 3'-5' exonucleolytically. DNA-binding requires protein and both gRNAs, as does nuclease activity. Cas9 recognizes the protospacer adjacent motif (PAM) in the CRISPR repeat sequences to help distinguish self versus nonself, as targets within the bacterial CRISPR locus do not have PAMs. DNA strand separation and heteroduplex formation starts at PAM sites; PAM recognition is required for catalytic activity.

關鍵字: GMP核酸酶;GMP Nuclease;Nuclease;ACRO;百普賽斯;
百普賽斯集團ACROBiosystems Group(股票代碼:301080)是成立于2010年的跨國生物科技公司��,是為全球生物醫(yī)藥��、健康產(chǎn)業(yè)領域提供關鍵生物試劑產(chǎn)品及解決方案的行業(yè)平臺型基石企業(yè)���。2021年在創(chuàng)業(yè)板上市����。百普賽斯集團業(yè)務遍布全球����,橫跨亞洲��、北美洲�、歐洲,在中國���、美國����、瑞士等12個城市設有辦公室�、研發(fā)中心及生產(chǎn)基地。目前累計服務客戶超6000家�����,與全球Top 20醫(yī)藥企業(yè)均建立了長期�����、穩(wěn)定的合作伙伴關系���。集團旗下?lián)碛衅放艫CROBiosystems百普賽斯�����、bioSeedin柏思薈��、Condense Capital墾拓資本和ACRODiagnostics百斯醫(yī)學等����。