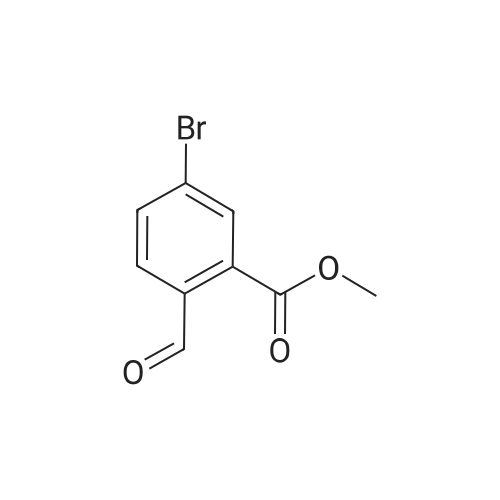
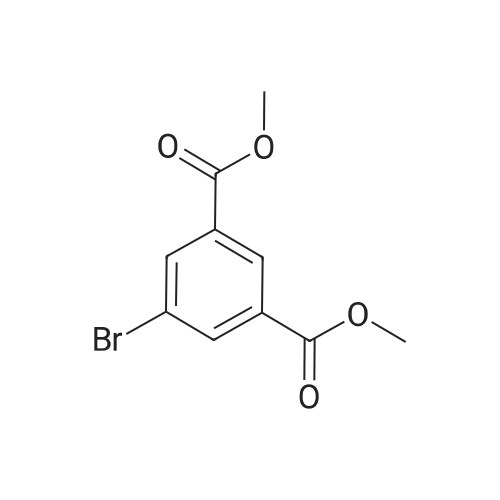
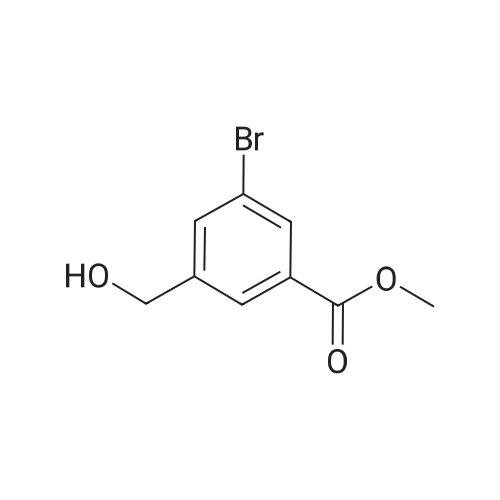
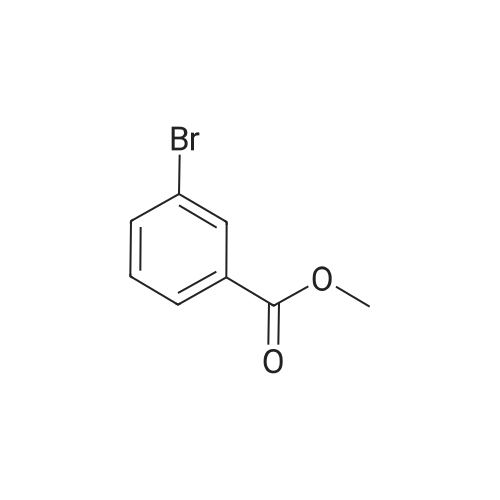
|
With potassium acetate;1,1'-bis-(diphenylphosphino)ferrocene; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In 1,4-dioxane; at 95℃; for 16h; |
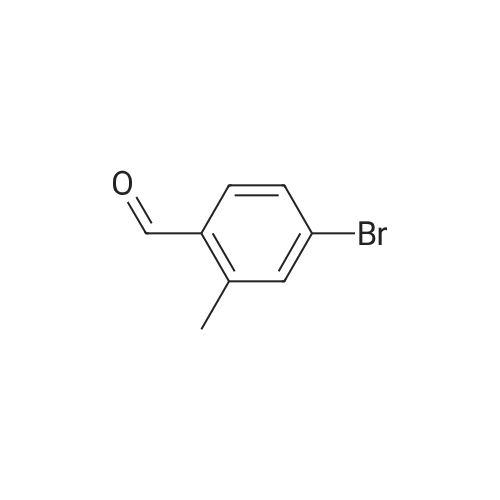
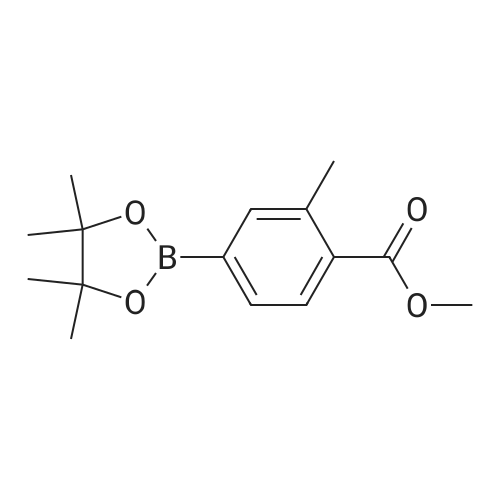
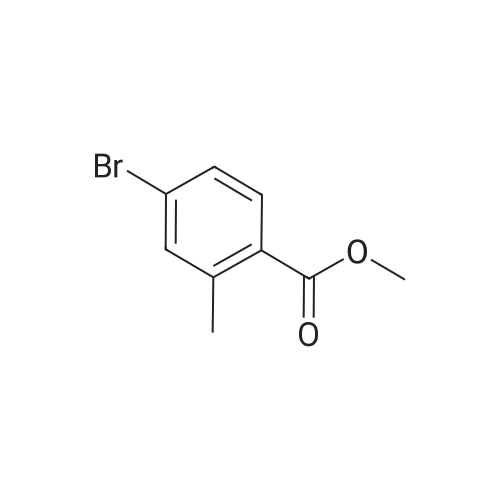
Preparation 17: 2-Methyl-4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)benzoic acid methyl ester4-Bromo-2-methylbenzoic acid methyl ester (0.028 mmol), ), 4,4,5,5,4',4',5',5'- octamethyl-[2,2']bi[[l,3,2]dioxaborolanyl] (0.028 mmol) and potassium acetate (0.064 mmol) were combined in dioxane (65 mL) and de-gassed with argon. [l,l'-Bis(diphenylphosphino)- ferrocene]dichloropalladium (5.62xlO~4 mmol) and l,l'-bis(diphenylphosphino)ferrocene (5.62xlO~4 mmol) were added and the mixture stirred at 950C under argon for 16 h. The mixture was cooled then partitioned between water and ethyl acetate. The water was separated and washed with ethyl acetate. The organic extracts were combined, dried (MgSO4), concentrated and purified by dry-flash chromatography to afford the title compound: RT = 4.07 min; m/z(ES+) = 277.24 [M+H]+ (LCMS protocol 3). |
|
With potassium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; In dimethyl sulfoxide; at 80℃; for 2h; |
Step A. Methyl 2-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzoate; A round bottomed flask was charged with methyl 4-bromo-2-methylbenzoate (3.98 g, 17.37 mmol), bis(pinacolato)diboron (4.85 g, 19.11 mmol), potassium acetate (5.12 g, 52.1 mmol), and dichloro[1,1'-bis(diphenylphosphino)ferrocene]palladium (II) dichloromethane adduct (0.426 g, 0.521 mmol). The flask was purged with nitrogen. Anhydrous DMSO (100 mL) was added, and the resulting suspension was degassed via nitrogen sparge. The mixture was then placed in a pre-heated oil bath (80 C.), and was held at this temperature for 2 h, whereupon it was allowed to cool to ambient temperature, then was poured into water. The aqueous phase was extracted with ether, and the organic phase was washed with brine. The organic phase was then separated, dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo. Purification by flash chromatography on silica gel (0 to 10% EtOAc in hexanes, then 10 to 100% EtOAc in hexanes) provided the title compound: LCMS m/z 277.6 [M+H]+; 1H NMR (500 MHz, CDCl3) δ 7.87 (d, J=7.5 Hz, 1H), 7.68 (s, 1H), 7.66 (d, J=7.5 Hz, 1H), 3.89 (s, 3H), 2.59 (s, 3H), 1.35 (s, 12H). |
|
With potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In dichloromethane; dimethyl sulfoxide; at 80℃; for 3h; |
Methyl 4-bromo-2-methylbenzoate (9.16 g) obtained in Step 2 was dissolved in dimethyl sulfoxide (120 ml), and [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane(1:1) (1.46 g), potassium acetate (11.8 g) and bis(pinacolato)diboron (11.2 g) were added at 80 C. The mixture was stirred for 3 hrs. The reaction mixture was cooled to room temperature, water was added and the mixture was extracted with ethyl acetate. The organic layer was washed successively with water and brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The obtained residue was purified by silica gel column chromatography (hexane:ethyl acetate=9:1) and the resulting compound was dissolved in toluene (80 ml) and ethanol (80 ml). Thereto was added a solution of [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II),complex with dichloromethane (1:1) (1.17 g) and (2R)-2-[((1R)-(2-bromophenyl)ethoxy)methyl]oxirane (10.5 g) obtained in Step 1 in ethanol (80 ml) and 2M aqueous sodium carbonate solution (80 ml) was added. The mixture was heated under reflux for 3 hrs. The reaction mixture was allowed to return to room temperature, and extracted with diethyl ether. The organic layer was washed successively with water and saturated brine, dried over sodium sulfate, and concentrated under reduced pressure. The obtained residue was purified by silica gel column chromatography (hexane:ethyl acetate=5:1) to give the title compound (8.93 g). 1H-NMR (300 MHz, δppm, CDCl3) 7.96(1H, d, J=8.6 Hz), 7.60(1H, d, J=6.7 Hz), 7.43-7.28(4H, m), 7.18-7.13(1H, m), 4.55(1H, q, J=6.4 Hz), 3.92(3H, s), 3.44-3.40(1H, m), 3.18-3.12(1H, m), 3.07-3.02(1H, m), 2.73-2.70(1H, m), 2.65(3H, s), 2.47-2.45(1H, m), 1.37(3H, d, J=6.4 Hz |
|
With potassium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; In dimethyl sulfoxide; at 80℃; for 2h;Inert atmosphere; |
Example 3; Step A. Methyl 2-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzoateA round bottomed flask was charged with methyl 4-bromo-2-methylbenzoate (3.98 g, 17.37 mmol), bis(pinacolato)diboron (4.85 g, 19.11 mmol), potassium acetate (5.12 g, 52.1 mmol), and dichloro [1,1'-bis(diphenylphosphino) ferrocene] palladium (II) dichloromethane adduct (0.426 g, 0.521 mmol). The flask was purged with nitrogen. Anhydrous DMSO (100 mL) was added, and the resulting suspension was degassed via nitrogen sparge. The mixture was then placed in a pre-heated oil bath (80 C.), and was held at this temperature for 2 h, whereupon it was allowed to cool to ambient temperature, then was poured into water. The aqueous phase was extracted with ether, and the organic phase was washed with brine. The organic phase was then separated, dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo. Purification by flash chromatography on silica gel (0 to 10% EtOAc in hexanes, then 10 to 100% EtOAc in hexanes) provided the title compound: LCMS m/z 277.6 [M+H]+; 1H NMR (500 MHz, CDCl3) δ 7.87 (d, J=7.5 Hz, 1H), 7.68 (s, 1H), 7.66 (d, J=7.5 Hz, 1H), 3.89 (s, 3H), 2.59 (s, 3H), 1.35 (s, 12H). |
|
With potassium acetate;palladium diacetate; In N,N-dimethyl-formamide; at 20 - 80℃; for 4.5h; |
As shown in Reaction Scheme 6, a mixture of bis(pinacolato)diboron (0.55 g, 2.2 mmol), methyl 4-bromo-2-methylbenzoate (0.50 g, 2.2 mmol), palladium(II) acetate (0.01 g, 0.07 mmol), and KOAc (0.64 g, 6.6 mmol) in DMF (7.5 mL) was degassed with argon for 30 min at rt. The mixture was then heated at 800C for 4 h. After cooling the mixture to rt, N-(4-bromo-2-fluorophenyl)-6- fluoro-l,3-benzothiazol-2-amine (0.74 g, 2.2 mmol), tetrakis(triphenylphosphine)palladium(0) (0.08 g, 0.07 mmol), and saturated aqueous NaHCO3 (5 mL) were added. The mixture was heated at 850C overnight. The mixture was poured into ice water and extracted with EtOAc. The combined organic phases were dried over Na2SO4, concentrated in vacuo, and purified by column chromatography (20% EtOAc in hexanes). This yielded 0.345 g (39%) of the title compound. EPO <DP n="28"/>LC/MS m/z 411.3 (MH+); retention time 4.15 min. 1H NMR (400 MHz, CD2Cl2) δ 2.62 (s, 3H), 3.90 (s, 3H), 7.10 (t, IH), 7.39-7.53 (m, 5H), 7.62-7.70 (m, IH), 7.99 (d, IH), 8.58 (t, IH). |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; at 85℃; for 6h;Inert atmosphere; |
Adding the compound 179a (500 mg, 2.18 mmol),Compound 179b (665.1 mg, 2.62 mmol), potassium acetate (427.8 mg, 4.36 mmol) and PdCl2 (dppf) (159.7 mg, 0.22 mmol) were dissolved in 1,4-dioxane (10 mL) and replaced with nitrogen three times. Then, it heated up to 85 C and stirred for 6 hours. Cool to room temperature,The reaction solution was directly used for feeding in the next step. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping