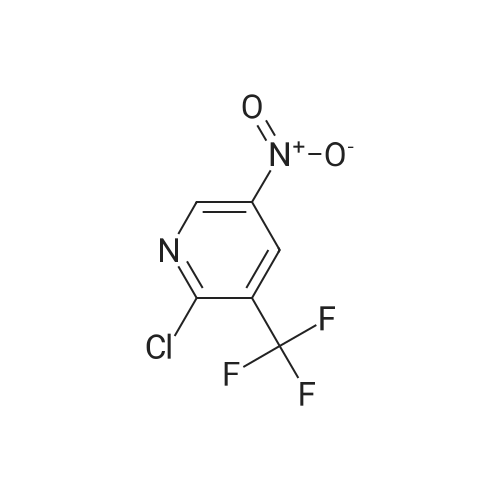
| 51.9% |
With iron; acetic acid; at 80℃; for 0.25h; |
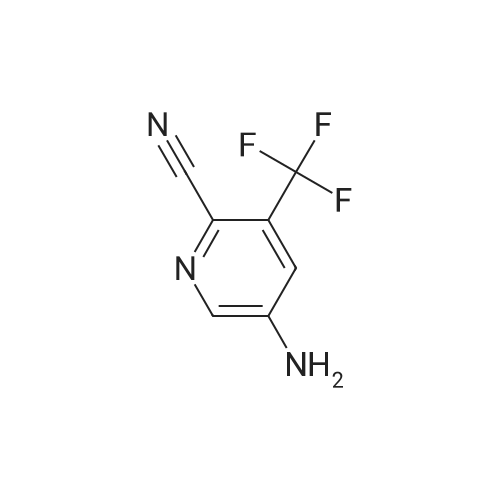
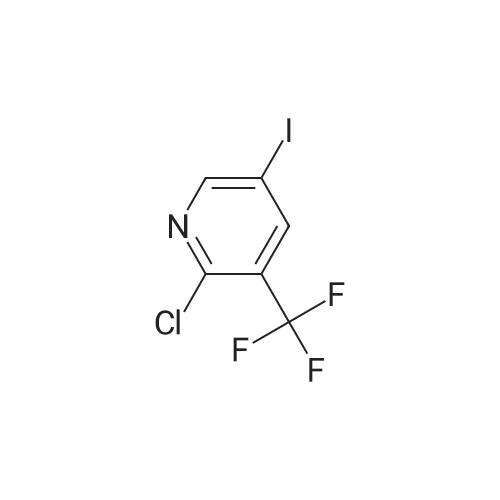
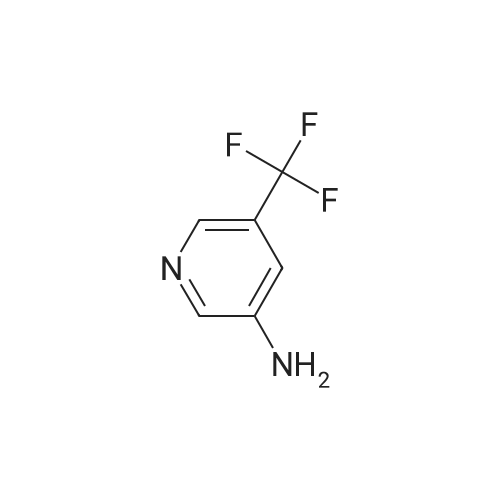
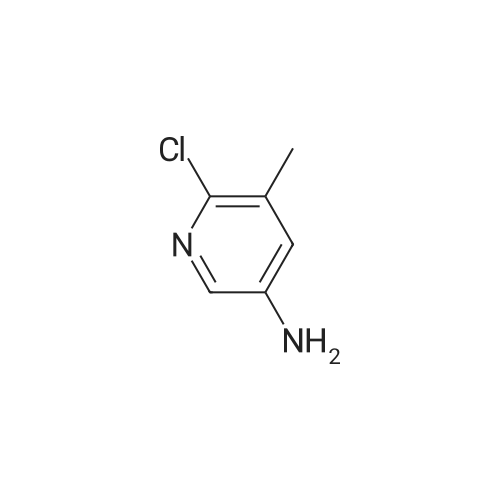
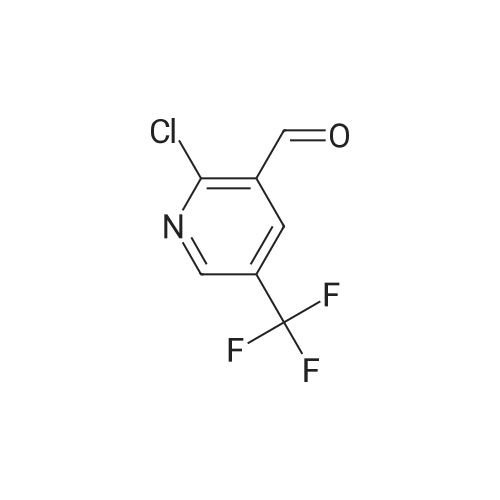
To a mixture of <strong>[99368-67-9]2-chloro-5-nitro-3-(trifluoromethyl)pyridine</strong> (2 g, 8.83 mmol) in acetic acid (10 mL) was added iron (2.465 g, 44.1 mmol) in one portion. The mixture was stirred at 80 C. for 15 min. The mixture was filtered and concentrated and then washed with aq. NaOH and extracted with EA. he crude material was purified by silica column chromatography (PE/EA=5:1). All fractions found to contain product by TLC (PE/EA=8:1, Rf=0.6) were combined and concentrated to yield a yellow solid of 6-chloro-5-(trifluoromethyl)pyridin-3-amine (1 g, 4.58 mmol, 51.9% yield): 1H NMR (400 MHz, CD3OD) delta 8.06 (s, 1H), 7.86 (d, J=8.60 Hz, 1H), 7.53 (d, J=8.60 Hz, 1H), 7.46-7.26 (m, 5H), 4.16-4.11 (m, 2H), 3.81 (s, 2H), 1.47 (t, J=6.62 Hz, 3H); ES-LCMS m/z 197.0 (M+H). |
| 51.9% |
With iron; acetic acid; at 80℃; for 0.25h; |
To a mixture of <strong>[99368-67-9]2-chloro-5-nitro-3-(trifluoromethyl)pyridine</strong> (2 g, 8.83 mmol) in acetic acid (10 inL) was added iron (2.465 g, 44.1 mmol) in one portion. The mixture was stirred at 80 C for 15 min. The mixture was filtered and concentrated and then washed with aqueous NaOH and extracted with EA. The crude material was purified by silica column chromatography (PE/EA = 5: 1). All fractions found to contain product by TLC (PE/EA = 8: 1, Rf = 0.6) were combined and concentrated to yield a yellow solid of 6-chloro-5-(trifluoromethyl)pyridin-3 -amine (1 g, 4.58 mmol, 51.9% yield): lH NMR (400 MHz, CD3OD) delta 8.06 (s, 1H), 7.86 (d, J = 8.60 Hz, 1H), 7.53 (d, J = 8.60 Hz, 1H), 7.46-7.26 (m, 5H), 4.16-4.11 (m, 2H), 3.81 (s, 2H), 1.47 (t, J = 6.62 Hz, 3H); ES-LCMS m/z 197.0 (M+H). |
| 51.9% |
With iron; acetic acid; at 80℃; for 0.25h; |
To a mixture of 2-chloro-5-nitro-3- (trifluoromethyl) pyridine (2 g 8.83 mmol) in AcOH (10 mL) was added iron (2.465 g 44.1 mmol) in one portion. The mixture was stirred at 80 for 15 min. The mixture was filtered and concentrated and then washed with aq. NaOH and extracted with EA. The residue was purified by silica column chromatography (PE/EA 51) . All fractions found to contain product by TLC (PE/EA 81 Rf 0.6) were combined and concentrated to yield a yellow solid of 6-chloro-5- (trifluoromethyl) pyridin-3-amine (1 g 4.58 mmol 51.9yield) 1HNMR(400 MHz CD3OD) delta 8.06 (s 1H) 7.86 (d J 8.60 Hz 1H) 7.53 (d J 8.60 Hz 1H) 7.46-7.26 (m 5H) 4.16-4.11 (m 2H) 3.81 (s 2H) 1.47 (t J 6.62 Hz 3H) ES-LCMS m/z 197.0 (M+H) |
|
With ethanol; water; iron; calcium chloride; for 1.0h;Heating / reflux; |
5. 6-Chloro-5-trifluorom.eihyl-pyridin-3-ylam.ine Heat a mixture of <strong>[99368-67-9]2-chloro-5-nitro-3-trifluoromethyl-pyridine</strong> (2.27 g, 10 mmol), calcium chloride (1.1 g, 10 mmol) and iron powder (4.5 g) in ethanol (30 mL) and water (5 mL) at reflux for 1 hour. Cool the mixture and filter through Celite. Evaporate the filtrate, dissolve the residue in EtOAc (200 mL) and wash with saturated NaHCO3(aq) (100 mL) and brine (100 mL). Dry the organic extract over MgSO4 and remove the solvent under reduced pressure to yield the title compound. |
|
With ammonium chloride; zinc; In ethanol; water; for 18.0h; |
EXAMPLE 27; N-[6-(3-Hydroxy-phenyl)-5-trifluoromethyl-pyridin-3-yl]-3-(4-trifluoromethyl-phenyl)-propionamide (Cpd 210) A. To a solution of compound 26c (1.478 g, 6.52 mmol) in 20 mL 4:1 EtOH/H2O was added NH4Cl (0.524 g, 9.80 mmol) and Zn powder (3.44 g, 52.6 mmol, <10 micron). The reaction was stirred under a nitrogen atmosphere for 18 hours then partitioned between 75 mL EtOAc and 75 mL H2O. This solvent mixture was filtered over a pad of Celite, 50 mL brine was added to help with the emulsion. The mixture was filtered again over Celite. The organic phase was isolated and dried over Na2SO4, filtered, and evaporated in vacuo to a residue. The residue was purified via silica gel chromatography (30-60% EtOAc/heptane) to give compound 27a as a yellow-orange powder. MS: M+H+=197.0, 1H NMR (d6-DMSO): delta 7.93 (d, 1H), 7.39 (d, 1H), 6.02 (s, 2H). |
|
With Raney-Ni; In tetrahydrofuran; for 24.0h; |
2-chloro-5-nitro-3-(trifluoromethyl) pyridine 4 (1.57g, 6.93mmol) was dissolved in tetrahydrofuran(THF) (10 ml), then was added to THF (20 ml) suspension of Raney -Ni (200 mg).Hydrogen gas was gentle foaming passing through a 24 hour stirring solutionusing a balloon. The mixture was filtered throughCelite (registered trademark) (World Minerals Inc., Lompoc, CA) and the solventwas evaporated under reduced pressure to obtain 6-chloro-5- (trifluoromethyl)pyridin-3-amine 5. |
|
With hydrogen; In tetrahydrofuran; at 22℃; for 24.0h; |
Synthesis of 6-chloro-5-(trifluoromethyl)pyridin-3-amine, 5 (0078) (0079) <strong>[99368-67-9]2-Chloro-5-nitro-3-(trifluoromethyl)pyridine</strong> 4 (1.57 g, 6.93 mmol) is dissolved in tetrahydrofuran (THF) (10 ml) and added to a suspension of Raney-Ni (200 mg) in THF (20 ml). Hydrogen gas is slowly bubbled through the stirred solution for 24 hours using a balloon. The mixture is filtered through Celite (available from World Minerals, Inc., Lompoc, Calif.) and the solvent is removed under reduced pressure to obtain 6-chloro-5-(trifluoromethyl)pyridin-3-amine 5. |
|
With iron; ammonium chloride; In methanol; water; at 20℃; for 4.0h; |
(0875) A mixture of iron (1.5 g) and ammonium chloride (2.38 g) in water (40 ml) was stirred at room temperature for 5 minutes. To this suspension was added Compound 288B in methanol (40 ml). The reaction mixture was stirred at room temperature for 1 hour. More iron (1.8 g) was added to the reaction mixture, and it was stirred for another 3 hours. The solid from the reaction mixture was filtered off, and the filtrate was partitioned between water and ethyl acetate. The combined organic layers were washed with brine, dried over MgSO4, filtered, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel eluting with 20percent ethyl acetate in hexanes to provide the title compound. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping