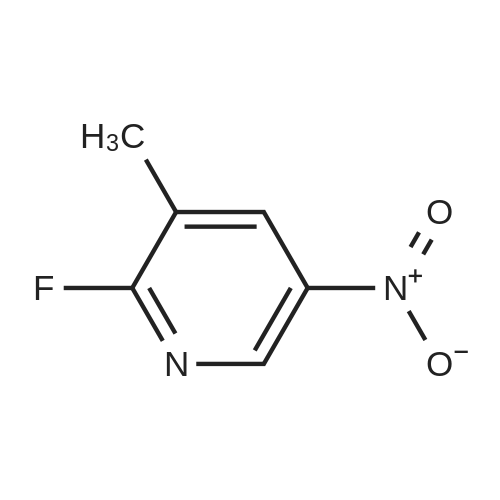
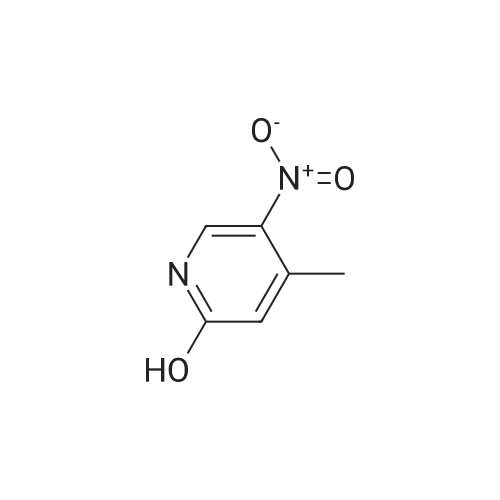
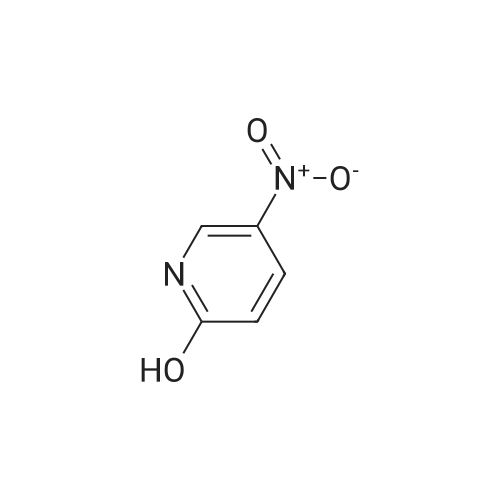
| 74% |
With sulfuric acid; nitric acid; at 20℃; |
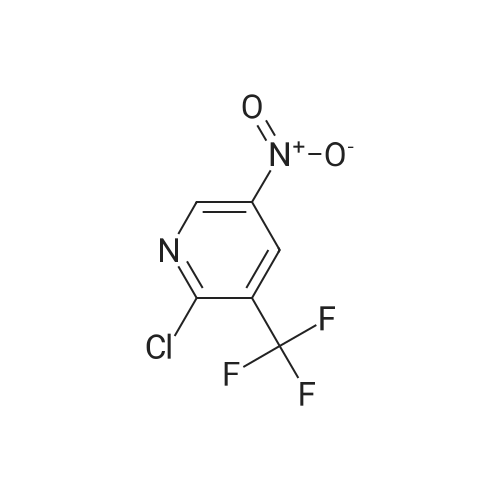
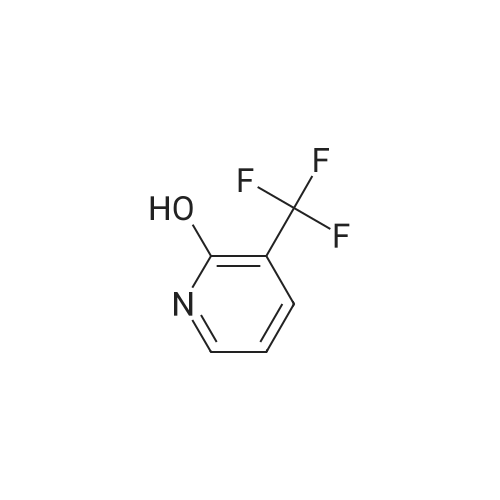
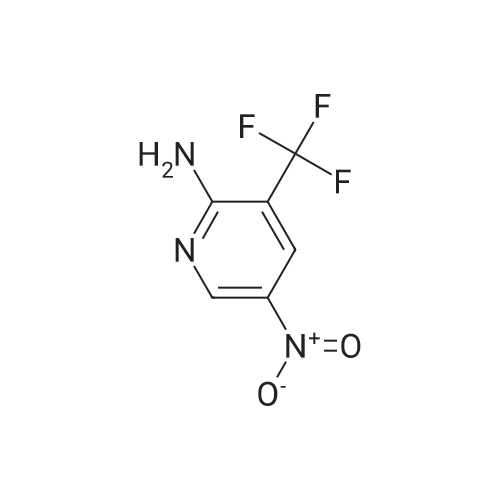
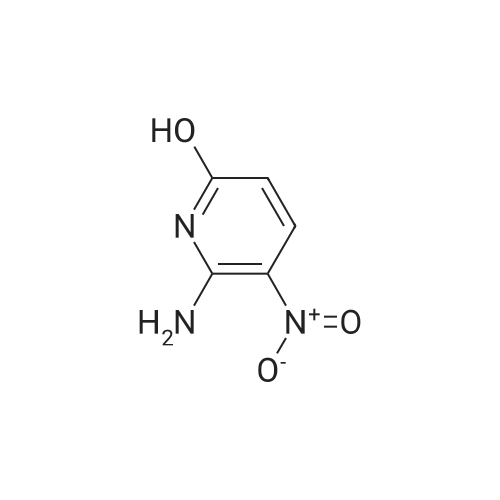
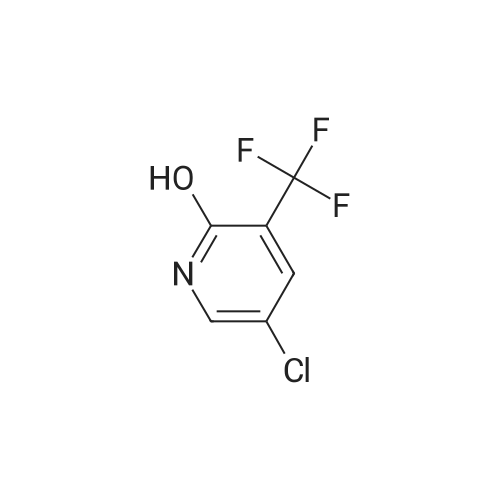
To a solution of compound 1 (25g, 0.15mol) in cocnH2SO4 (100ml) was added the mixture HNO3 and cocnH2SO4 (v/v=1/1)(100ml) drop-wise at room temperature. The reaction mixture was stirred for 4h. This reaction mixture was added into ice (1kg) portion-wise, stirred for 2h, filtrated, washed with water (50ml), then, dried to afford 5-nitro-3-(trifluoromethyl)pyridin-2-ol (2) (23.3g, 74%). 1H NMR (400 MHz, DMSO-d6) delta 13.49 (br, 1H), 8.97 (s, 1H), 8.47 (s, 1H). 13C NMR (101 MHz, DMSO-d6) delta 158.0, 142.4, 134.1 (q, J =5.1Hz), 128.7, 121.9 (q, J = 272.7 Hz), 116.8 (q, J = 30.3 Hz). |
| 73.3% |
With sulfuric acid; nitric acid; at 0 - 60℃; for 21h; |
To a mixture of 3- (trifluoromethyl) pyridin-2-ol (2 g 12.26 mmol) was added nitric acid (1.644 mL 36.8 mmol) and H2SO4(12.03 g 123 mmol) at 0 . Then the mixture was stirred at 25 for 16 h. The mixture was then warmed to 60 for 5 h cooled and added to 150 g of ice. The mixture was extracted with EA (2 x 100 mL) and washed with H2O (100 mL) to give the organic layer. The combined organic extract was washed with brine dried over Na2SO4 concentrated to yield a brown solid of 5-nitro-3- (trifluoromethyl) pyridin-2-ol (2.2 g 8.99 mmol 73.3yield) 1HNMR(400 MHz CD3OD) delta 8.91 (d J 2.43 Hz 1H) 9.42 (d J 2.43 Hz 1H) ES-LCMS m/z 209.0 (M+H) |
| 69% |
|
Reference Example 117 5-nitro-3-(trifluoromethyl)pyridin-2-ol; 2-Hydroxy-3-(trifluoromethyl)pyridine (3.0 g) was added to conc. sulfuric acid (18 mL) under ice-cooling, and the mixture was stirred at the same temperature for 5 min. Fuming nitric acid (90-95%, 7 mL) was added dropwise over 5 min, and the mixture was allowed to return to room temperature over 2 hr, heated to 50 C. and stirred for 3 hr. After cooling to room temperature, the reaction mixture was poured into ice (200 g), and the mixture was extracted with ethyl acetate. The extract was washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The precipitate was washed with diisopropyl ether to give the title compound as a solid (yield 2.7 g, 69%). 1H-NMR (CDCl3) delta: 8.65-8.67 (1H, m), 8.80-8.81 (1H, m), 1H not detected. |
| 51.5% |
With sulfuric acid; nitric acid; at 26℃; for 10.5h;Cooling with ice; |
To an ice-cooled solution of 3-(trifluoromethyl)pyridin-2-ol (4 g, 24.53 mmol) in H2SO4 (26.1 mL, 491 mmol) was added nitric acid (1.206 mL, 27.0 mmol) dropwise. After 30 min, the ice bath was removed and the reaction was stirred at 26 C. for 10 h. The reaction mixture was added to 120 g ice. The resulting precipitate was collected by filtration, rinsed with additional H2O and air-dried to afford the first batch of product. Another crop of product was obtained after evaporating the mother liquor to less than 100 mL, cooling on an ice bath, and adding NaOH to adjust to pH=8. The mixture was extracted by EA (100 mL). The organic layer was dried and concentrated to give the product, which was combined with the first batch to yield a yellow solid of 5-nitro-3-(trifluoromethyl)pyridin-2-ol (2.63 g, 12.64 mmol, 51.5% yield): 1H NMR (400 MHz, CD3OD) delta: 8.86 (d, J=3.1 Hz, 1H), 8.55 (d, J=2.6 Hz, 1H); ES-LCMS m/z 209.0 (M+H). |
|
With sulfuric acid; nitric acid; In water; at 0 - 20℃; for 3h; |
3. 5-Nitro-3-trifluoromethyl-pyridin-2-ol To 3-trifluoromethyl-pyridin-2-ol (1.63 g, 10 mmol) in concentrated sulfuric acid at O0C, add dropwise fuming nitric acid (2 mL). Stir the mixture at room temperature for 3 hours and pour onto ice. Collect the precipitate by filtration, air-dry and finally dry in a vacuum oven overnight to give the title compound as a white solid |
|
|
EXAMPLE 26; 3-(4-tert-Butyl-phenyl)-N-(6-imidazol-1-yl-5-trifluoromethyl-pyridin-3-yl)-propionamide (Cpd 202) A. To an ice-cooled solution of compound 26a (5.93 g, 36.4 mmol) in 35 mL concentrated H2SO4 was added concentrated HNO3 (2.6 mL, 40.8 mmol) dropwise. After 30 minutes the ice bath was removed and the reaction was stirred at ambient temperature for 15 hours. The reaction mixture was then warmed to 60 C for 5 hours, cooled, and added to 150 g of ice. The resulting precipitate was collected by filtration, rinsed with additional water, and air-dried to afford the first batch of product. Another crop of product was obtained after evaporating the mother liquor to less than 100 mL volume, cooling on an ice bath, and adding NaOH (25.34 g). This solid was filtered off, rinsed with water, and air-dried to provide another batch of product of compound 26b. 1H NMR (d6-DMSO): delta 13.49 (br s, 1H), 8.96 (s, 1H), 8.47 (s, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













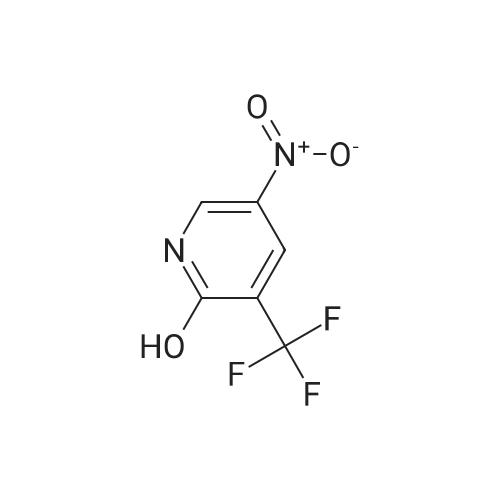

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping