Alternatived Products of [ 98-91-9 ]
Product Details of [ 98-91-9 ]
| CAS No. : | 98-91-9 |
MDL No. : | MFCD00004852 |
| Formula : |
C7H6OS
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | UIJGNTRUPZPVNG-UHFFFAOYSA-N |
| M.W : |
138.19
|
Pubchem ID : | 7414 |
| Synonyms : |
|
Application In Synthesis of [ 98-91-9 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 98-91-9 ]
- 1
-
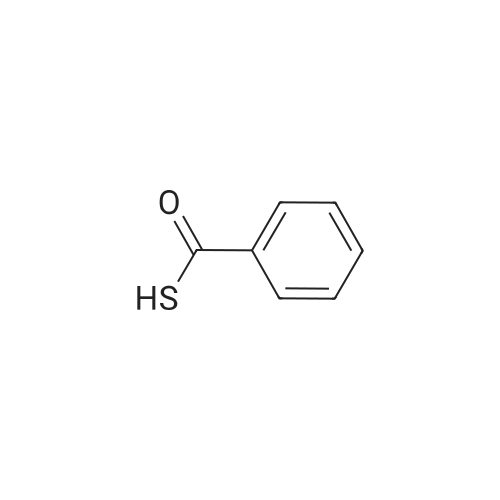 [ 98-91-9 ]
[ 98-91-9 ]

-
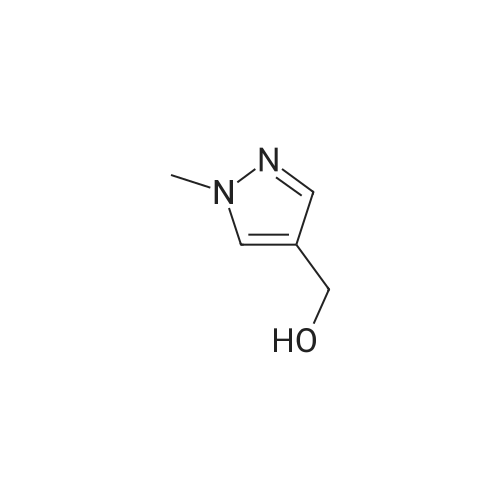 [ 112029-98-8 ]
[ 112029-98-8 ]

-
 [ 163008-92-2 ]
[ 163008-92-2 ]
- 2
-
 [ 98-91-9 ]
[ 98-91-9 ]

-
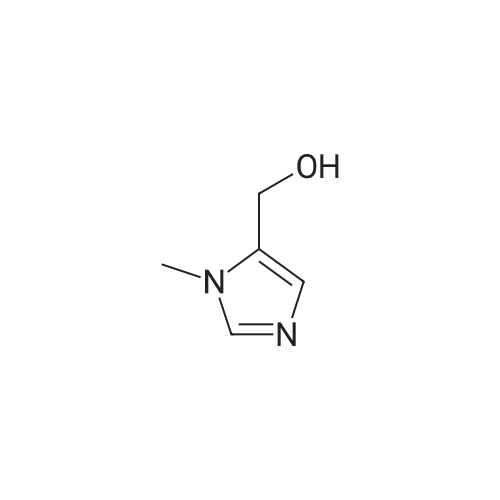 [ 38993-84-9 ]
[ 38993-84-9 ]

-
 [ 163009-15-2 ]
[ 163009-15-2 ]
- 3
-
 [ 98-91-9 ]
[ 98-91-9 ]

-
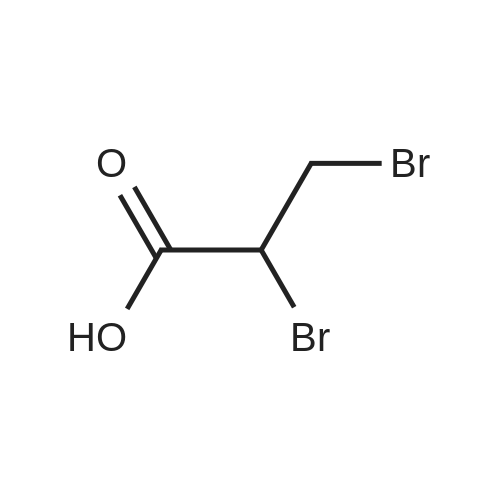 [ 600-05-5 ]
[ 600-05-5 ]

-
 [ 7664-93-9 ]
[ 7664-93-9 ]

-
2,3-bis-(benzoylthio)-propionic acid
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 117.8 g (68%) |
With potassium carbonate; In (2S)-N-methyl-1-phenylpropan-2-amine hydrate; toluene; |
(a) Preparation of the starting material 115.9 g (0.5 mole) of <strong>[600-05-5]2,3-dibromopropionic acid</strong> and 138.2 g (1.0 mole) of thiobenzoic acid were added to a thoroughly stirred suspension, cooled to 0 C., of 276.4 g (2.0 moles) of anhydrous, powdered potassium carbonate in 700 ml of toluene. The mixture was stirred for a short time at 0 C., after which its temperature was allowed to increase slowly. At about 20 C., an exothermic reaction took place spontaneously; the reaction temperature was kept below 40 C. by cooling externally with ice water. When the reaction was complete, the mixture was diluted with 1 liter of toluene and then decanted, and the residue was digested once again with toluene and then introduced into 1 liter of ice water. After acidification with aqueous 2N H2 SO4, the product was extracted with twice 500 ml of ethyl acetate. The organic phases were dried with anhydrous sodium sulfate and then evaporated down under reduced pressure. Crystallization from carbon tetracloride/cyclohexane gave 117.8 g (68%) of 2,3-bis-(benzoylthio)-propionic acid of melting point 116-118 C. (Rf=0.41 in system II). |
- 4
-
 [ 98-91-9 ]
[ 98-91-9 ]

-
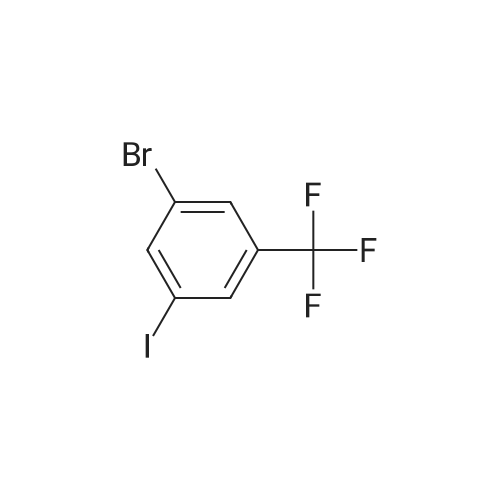 [ 481075-59-6 ]
[ 481075-59-6 ]

-
 [ 1215077-33-0 ]
[ 1215077-33-0 ]
| Yield | Reaction Conditions | Operation in experiment |
| 82% |
With N-ethyl-N,N-diisopropylamine;copper(l) iodide; 1,10-Phenanthroline; In toluene; at 110℃; for 24.0h;Inert atmosphere; |
Step 1: To a solution of <strong>[481075-59-6]1-bromo-3-iodo-5-(trifluoromethyl)benzene</strong> (55) (2.0 g, 5.7 mmol), thiobenzoic acid (56), (0.67 ml, 5.7 mmol), 1,10-phenanthroline (0.21 g, 1.08 mmol) in toluene were added DIPEA (2 ml) and CuI (0.11 g, 0.57 mmol). The resulting mixture was degassed by bubbling argon for 2 min and stirred at 110 C. for 24 hrs under argon. The reaction mixture was filtered through Celite and concentrated under reduced pressure. Purification by flash chromatography (5% to 15% EtOAc hexanes gradient) gave benzothioate 57 as a light yellow oil. Yield (1.7 g, 82%); 1H NMR (400 MHz, CD3OD) delta 7.96-8.04 (m, 4H), 7.80-7.83 (m, 1H), 7.69 (tt, J=6.0, 1.6 Hz, 1H), 7.53-7.58 (m, 2H). |
- 5
-
 [ 98-91-9 ]
[ 98-91-9 ]

-
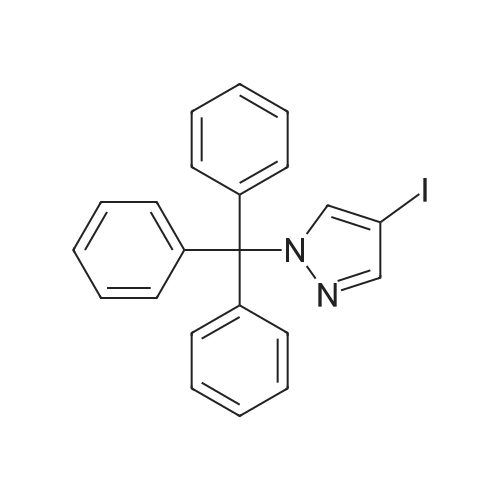 [ 191980-54-8 ]
[ 191980-54-8 ]

-
 [ 1269071-49-9 ]
[ 1269071-49-9 ]
| Yield | Reaction Conditions | Operation in experiment |
| 3.88 g |
With copper(l) iodide; 1,10-Phenanthroline; N-ethyl-N,N-diisopropylamine; In toluene; for 16h;Inert atmosphere; Reflux; |
To a solution of 4-iodo-l-trityl-pyrazole (3.48 g, 1 equiv.), thiobenzoic acid (1.12 mL, 1.2 equiv.), 1, 10-phenantroline (0.22 mL, 0.2 equiv.) and D/PEA (2.78 mL, 2 equiv.) in toluene (16 mL) was added Cul (0.15 g, 0.1 equiv.). The resulting mixture was degassed and then stirred under argon at reflux temperature for 16 h. The reaction mixture was filtered through a pad of Celite, and the pad was washed with toluene. The solvent was removed under vacuum, and the crude product was purified by flash chromatography on silica gel (eluting with a cyclohexane/EtOAc gradient, 0-10 % of EtOAc) to afford the expected product (3.88 g). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping












