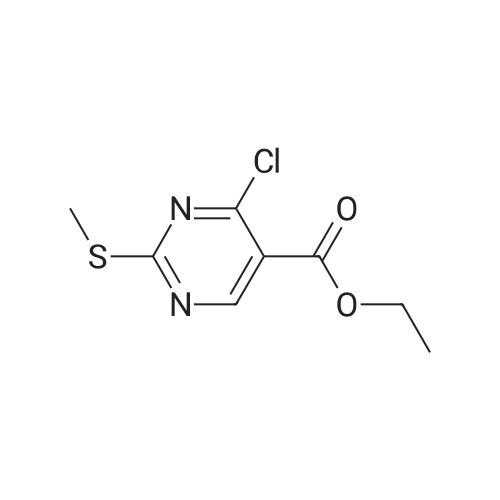
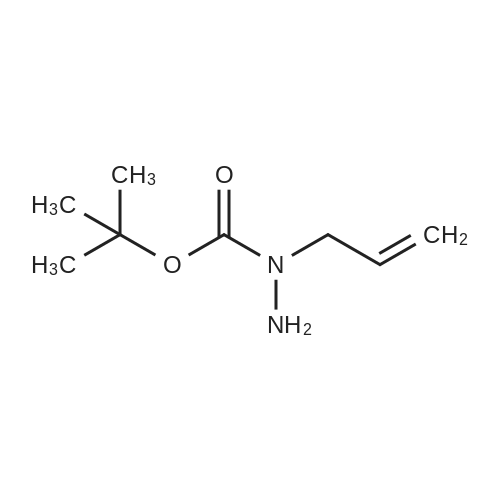
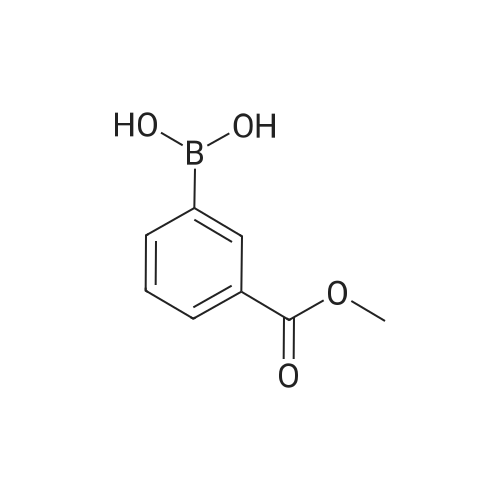
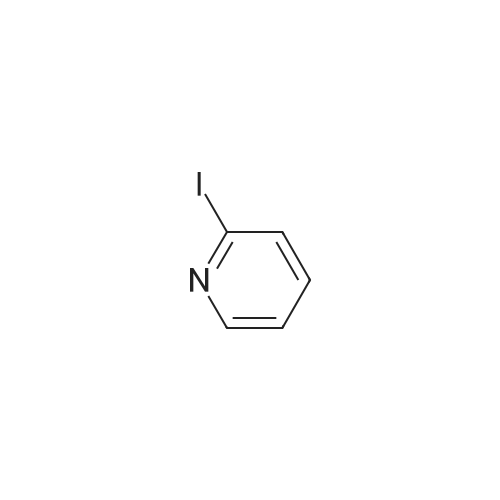
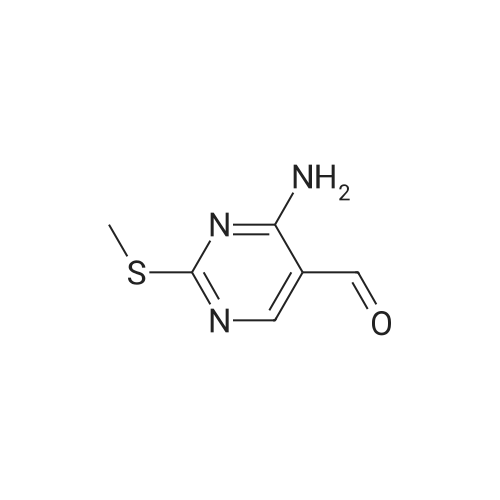
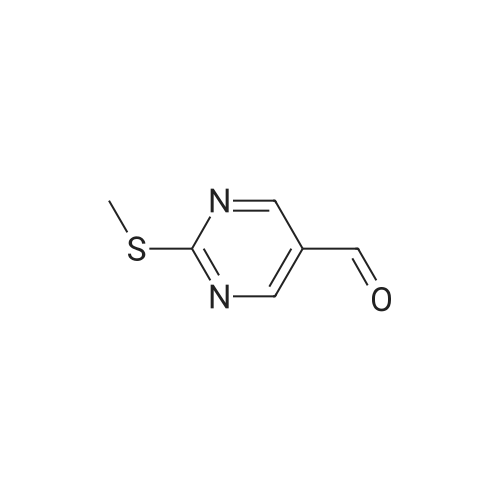
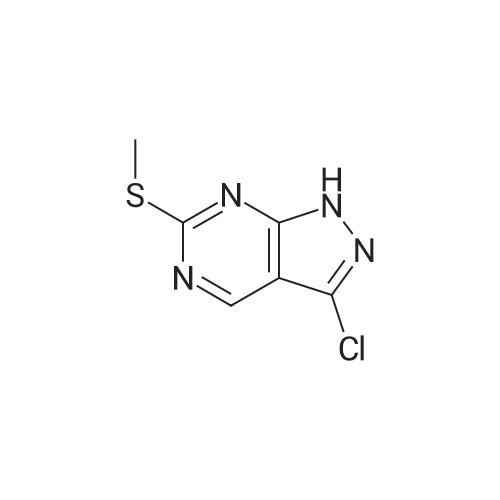
|
With potassium carbonate; N,N`-dimethylethylenediamine;copper(l) iodide; at 95℃; |
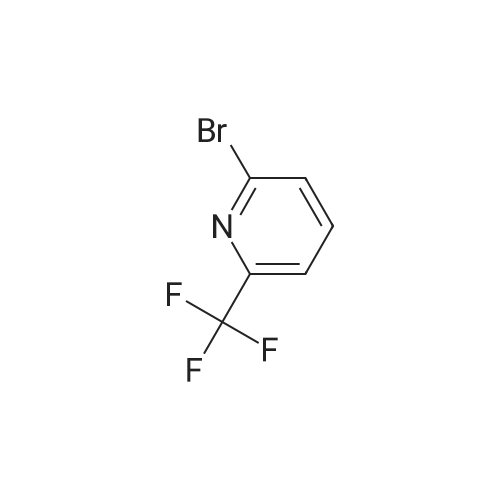
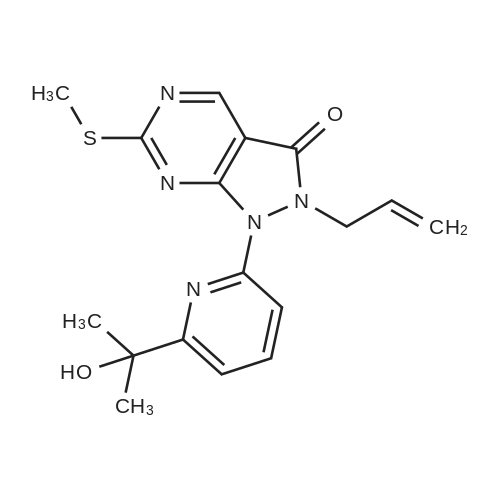
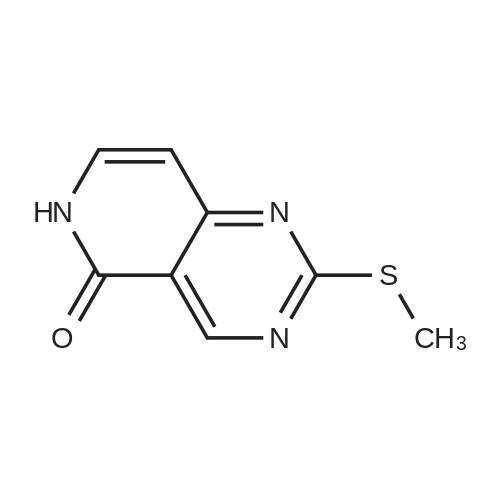
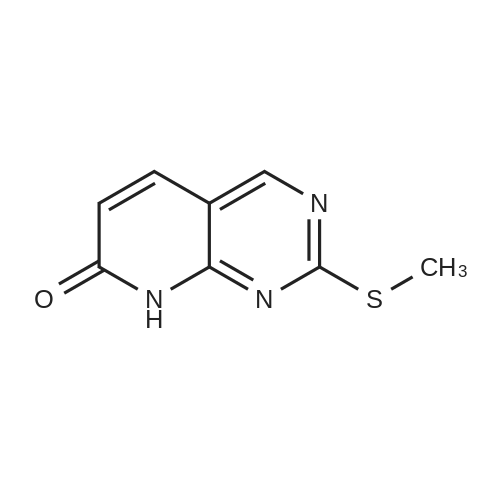
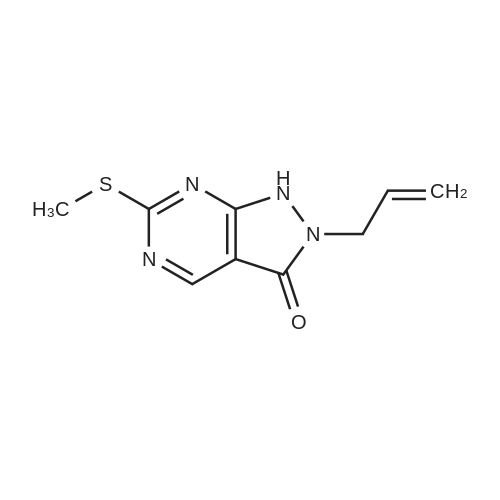
1) Production of 2-allyl-6-(methylthio)-1-pyridin-2-yl-3H-pyrazolo[3,4-d]pyrimidin-3-one 2.4 mL of N,N'-dimethylethylenediamine was added to 1,4-dioxane (50 mL) solution of 4.44 g of <strong>[955368-90-8]2-allyl-6-(methylthio)-1,2-dihydro-3H-pyrazolo[3,4-d]pyrimidin-3-one</strong>, 3.80 g of copper(I) iodide, 5.33 g of 2-iodopyridine and 3.80 g of potassium carbonate, and stirred overnight at 95 C. The reaction liquid was cooled, aqueous ammonia was added thereto and extracted with ethyl acetate, washed with saturated saline water and dried with anhydrous magnesium sulfate. The solvent was evaporated away under reduced pressure, and crystallized with ethyl acetate to obtain 5.15 g of the entitled compound as a white solid. 1H-NMR (400 MHz, CDCl3) delta: 8.94 (1H, s), 8.52 (1H, d, J=5.1 Hz), 7.90 (2H, d, J=3.5 Hz), 7.29-7.25 (1H, m), 5.68 (1H, ddt, J=17.0, 10.2, 6.3 Hz), 5.05 (1H, d, J=10.2 Hz), 4.91 (1H, d, J=17.0 Hz), 4.85 (1H, d, J=6.3 Hz), 2.58 (3H, s). ESI-MS Found: m/z[M+H]+ 300. |
|
With copper(l) iodide; potassium carbonate; N,N`-dimethylethylenediamine; In 1,4-dioxane; at 95℃; |
Reference Example 1 :Production of 2-allyl-6-fmethylthio)-l-pyridin-2-yl-3H-pyrazolo[3,4-d1pyrimidin-3-one2.4 mL of N,N'-dimethyl ethyl enediamine was added to 1,4-dioxane (50 mL) solution of 4.44 g of 2-allyl-6-(methylthio)-l,2-dihydro-3H-pyrazolo[3,4-d]pyrimidin-3-one, 3,80 <n="30"/>g of copper(I) iodide, 5.33 g of 2-iodopyridine and 3.8O g of potassium carbonate, and stirred overnight at 95C. The reaction liquid was cooled, aqueous ammonia was added thereto and extracted with ethyl acetate, washed with saturated saline water and dried with anhydrous magnesium sulfate. The solvent was evaporated away under reduced pressure, and crystallized with ethyl acetate to obtain 5.15 g of the entitled compound as a white solid. 1 H-NMR (400 MHz, CDCI3) delta: 8.94 (IH, s), 8.52 (IH, d, J=5.1 Hz), 7.90 (2H, d, J=3.5 Hz),7.29-7.25 (IH, m), 5.68 (IH, ddt, J=I 7.0, 10.2, 6.3 Hz), 5.05 (IH, d, J=10.2 Hz), 4.91 (IH, d, J=I 7.0 Hz), 4.85 (IH, d, J=6.3 Hz), 2.58 (3H, s). ESI-MS Found: m/z[M+H]+ 300. |
|
With potassium carbonate;copper(l) iodide; N,N`-dimethylethylenediamine; In 1,4-dioxane; at 95℃; |
1) Production of 2-allyl-6-(methylthio)-1-pyridin-2-yl-3H-pyrazolo[3,4-d]pyrimidin-3-one: N,N'-dimethylethylenediamine (2.4 mL) was added to a 1,4-dioxane (50 mL) solution of <strong>[955368-90-8]2-allyl-6-(methylthio)-1,2-dihydro-3H-pyrazolo[3,4-d]pyrimidin-3-one</strong> (4.44 g), copper(I) iodide (3.80 g), 2-iodopyridine (5.33 g) and potassium carbonate (3.80 g), and stirred overnight at 95C. The reaction liquid was cooled, then aqueous ammonia was added thereto and extracted with ethyl acetate, washed with saturated saline water, and dried with anhydrous magnesium sulfate. The solvent was evaporated away under reduced pressure, and the residue was crystallized with ethyl acetate to give the entitled compound as a white solid (5.15 g). 1H-NMR (400 MHz, CDCl3) delta: 8.94 (1H, s), 8.52 (1H, d, J = 5.1 Hz), 7.90 (2H, d, J = 3.5 Hz), 7.29-7.25 (1H, m), 5.68 (1H, ddt, J = 17.0, 10.2, 6.3 Hz), 5.05 (1H, d, J = 10.2 Hz), 4.91 (1H, d, J = 17.0 Hz), 4.85 (2H, d, J = 6.3 Hz), 2.58 (3H, s). ESI-MS Found: m/z[M+H]+ 300. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping