|
With sodium t-butanolate;palladium diacetate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; In toluene; at 80℃; for 14h; |
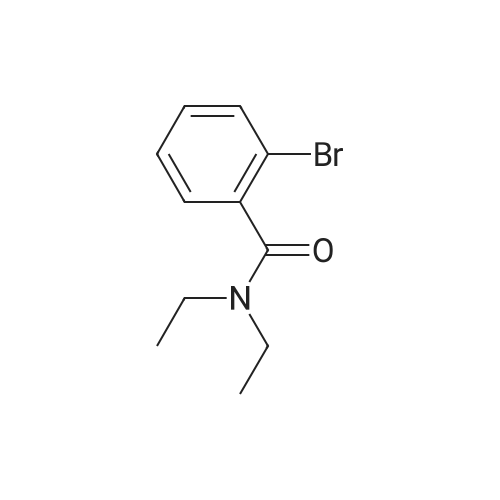
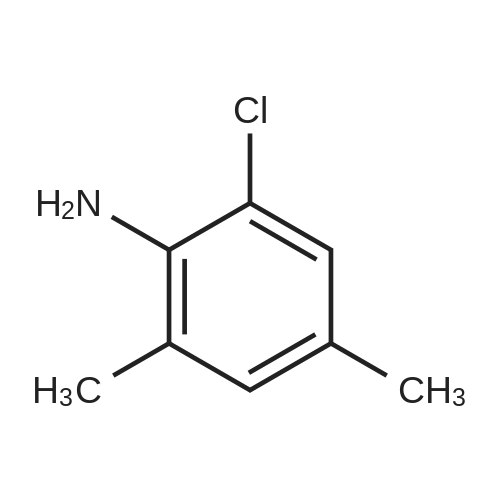
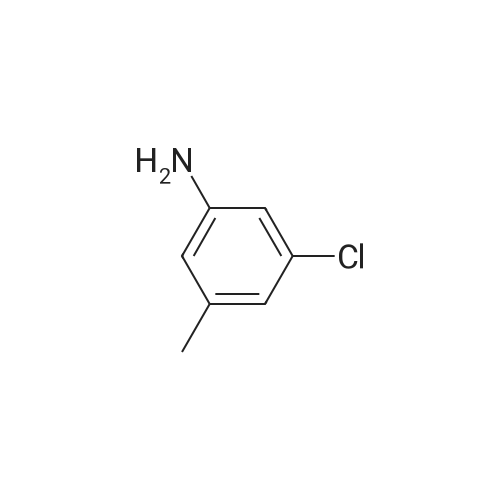
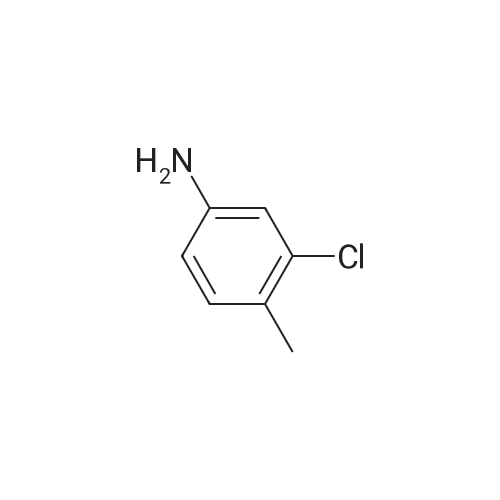
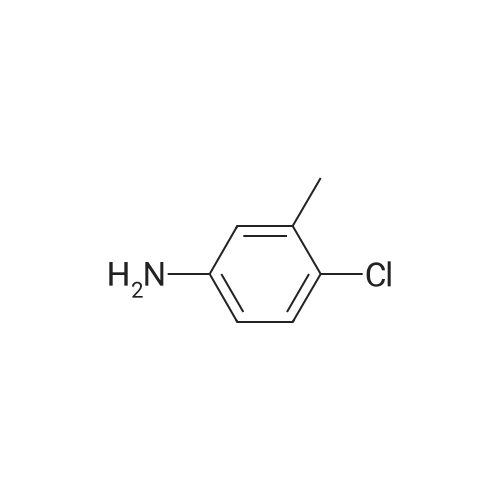
To a mixture of N, N-diethyl (2-bromobenzyl) amide (189JO10) (1.41 g, 5.50 mmol) and 4-chloro-2-methylaniline (1.01 g, 7.15 mmol) in deoxygenised toluene (14 mL) was added NaOtBu (0.74 g, 7.7 mmol), rac-BINAP (110 mg, 0.17 mmol) and Pd (OAc) 2 (18 mg, 0.08 mmol) and the resulting mixture was stirred under Ar for 14 h at 80C. The mixture was filtered through celite, concentrated and flash chromatographed (Si02, heptane: EtOAc, 10: 1-4: 1) which gave unprotected intermediate ketone (1.50 g) containing about 15% impurities. [0267] The mixture containing the intermediate was dissolved in DMF (20 mL). p-Methoxybenzyl chloride (0.90 mL, 6.6 mmol) was added and then NaH (0.23 g, 5.6 mmol, 60% in mineral oil) was added portions-wise. The resulting mixture was stirred at room temperature for 1 h, and then quenched by addition of saturated aqueous NaHC03- solution. The mixture was diluted with EtOAc, washed with saturated aqueous NaHC03- solution, dried (Na2S04), concentrated and flash chromatographed (Si02, toluene: EtOAc 10: 1) to give 1.66 g (68%) of the title compound (189JO26). lH NMR (CDCl3) 6 7. 35 (m, 2 H), 7.20 (m, 1 H), 7.09-6. 99 (m, 4 H), 6.91 (m, 2 H), 6.80 (m, 2 H), 4.84/4. 54 (Abq, 2 H, J = 16. 2 Hz), 3.74 (s, 3H), 3.18 (m, 2H), 3.03 (m, 1 H), 2,48 (m, 1 H), 2.17 (s, 3 H), 1.01 (t, 3 H, J= 7.2 Hz), 0.97 (t, 3 H, J= 7.0 Hz), 13C NMR (CDCl3) S 169.6, 158.7, 146.53, 146.51, 137.0, 131.3, 130.9, 130.4, 129.6, 129.3, 128. 7,127. 8,127. 4,126. 3,122. 8,121. 4,114. 0, 57. 1, 55. 3,43. 3, 39. 0,19. 1,13. 9,12. 9. MS (ESI) 437 (MH+). |
|
With racemic-2,2'-bis(diphenylphosphino)-1,1'-binaphthyl; sodium t-butanolate;palladium diacetate; In toluene; at 80℃; for 14h; |
To a mixture of W,N-diethyl(2-bromobenzyl)amide (189JO10) (1.41 g, 5.50 mmol) and 4-chloro-2-methylaniline (1.01 g, 7.15 mmol) in deoxygenised toluene (14 mL) was added NaO1Bu (0.74 g, 7.7 mmol), rac-BINAP (110 mg, 0.17 mmol) and Pd(OAc)2 (18 mg, 0.08 mmol) and the resulting mixture was stirred under Ar for 14 h at 8O0C. The mixture was filtered through celite, concentrated and flash chromatographed (SiO2, heptane: EtOAc, 10: 1-4: 1) which gave unprotected intermediate ketone (1.50 g) containing about 15% impurities.[0269] The mixture containing the intermediate was dissolved in DMF (20 mL). /7-Methoxybenzyl chloride (0.90 mL, 6.6 mmol) was added and then NaH (0.23 g, 5.6 mmol, 60% in mineral oil) was added portions-wise. The resulting mixture was stirred at room temperature for 1 h, and then quenched by addition of saturated aqueous NaHCθ3-solution. The mixture was diluted with EtOAc, washed with saturated aqueous NaHCO 3-solution, dried (Na2SO-O, concentrated and flash chromatographed (SiO2, toluene: EtOAc 10:1) to give 1.66 g (68%) of the title compound (189JO26). 1H NMR (CDCl3) δ 7.35 (m, 2 H), 7.20 (m, 1 <n="95"/>H), 7.09-6.99 (m, 4 H), 6.91 (m, 2 H), 6.80 (m, 2 H), 4.84 /4.54 (Abq, 2 H, J = 16.2 Hz), 3.74 (s, 3H), 3.18 (m, 2H), 3.03 (m, 1 H), 2,48 (m, 1 H), 2.17 (s, 3 H), 1.01 (t, 3 H, J= 7.2 Hz), 0.97 (t, 3 H, J = 7.0 Hz), 13C NMR (CDCl3) δ 169.6, 158.7, 146.53, 146.51, 137.0, 131.3, 130.9, 130.4, 129.6, 129.3, 128.7, 127.8, 127.4, 126.3, 122.8, 121.4, 114.0, 57.1, 55.3, 43.3, 39.0, 19.1, 13.9, 12.9. MS (ESI) 437 (MH+). |
|
With sodium t-butanolate;palladium diacetate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; In toluene; at 80℃; for 14h; |
To a mixture of N,N-diethyl(2-bromobenzyl)amide (189JO10) (1.41 g, 5.50 mmol) and 4-chloro-2-methylaniline (1.01 g, 7.15 mmol) in deoxygenised toluene (14 mL) was added NaO4Bu (0.74 g, 7.7 mmol), rac-BINAP (110 mg, 0.17 mmol) and Pd(OAc)2 (18 mg, 0.08 mmol) and the resulting mixture was stirred under Ar for 14 h at 8O0C. The mixture was filtered through celite, concentrated and flash chromatographed (SiO2, heptane:EtOAc, 10:1-4:1) which gave unprotected intermediate ketone (1.50 g) containing about 15% impurities. EPO <DP n="112"/>[0304] The mixture containing the intermediate was dissolved in DMF (20 mL). p-Methoxybenzyl chloride (0.90 mL, 6.6 mmol) was added and then NaH (0.23 g, 5.6 mmol, 60% in mineral oil) was added portions-wise. The resulting mixture was stirred at room temperature for 1 h, and then quenched by addition of saturated aqueous NaHCO3-solution. The mixture was diluted with EtOAc, washed with saturated aqueous NaHCO3-solution, dried (Na2SO4), concentrated and flash chromatographed (SiO2, toluene: EtOAc 10:1) to give 1.66 g (68%) of the title compound (189JO26). 1H NMR (CDCl3) δ 7.35 (m, 2 H), 7.20 (m, 1 H), 7.09-6.99 (m, 4 H), 6.91 (m, 2 H), 6.80 (m, 2 H), 4.84 /4.54 (Abq, 2 H, J = 16.2 Hz), 3.74 (s, 3H), 3.18 (m, 2H), 3.03 (m, 1 H), 2,48 (m, 1 H), 2.17 (s, 3 H), 1.01 (t, 3 H, J= 7.2 Hz), 0.97 (t, 3 H, J = 7.0 Hz), 13C NMR (CDCl3) δ 169.6, 158.7, 146.53, 146.51, 137.0, 131.3, 130.9, 130.4, 129.6, 129.3, 128.7, 127.8, 127.4, 126.3, 122.8, 121.4, 114.0, 57.1, 55.3, 43.3, 39.0, 19.1, 13.9, 12.9. MS (ESI) 437 (MH+). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +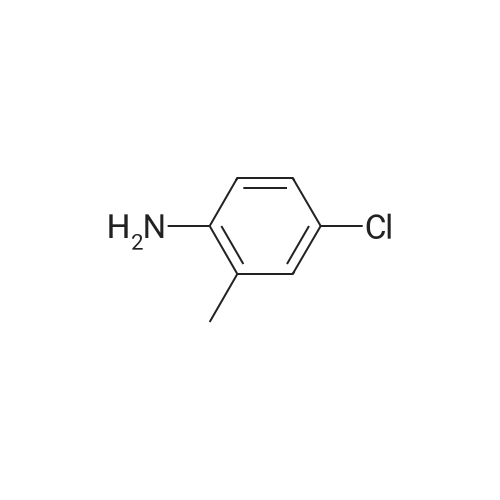

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping