Alternatived Products of [ 93413-76-4 ]
Product Details of [ 93413-76-4 ]
| CAS No. : | 93413-76-4 |
MDL No. : | MFCD06658142 |
| Formula : |
C15H19NO2
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | ASYJSBPNAIDUHX-UHFFFAOYSA-N |
| M.W : |
245.32
|
Pubchem ID : | 9899621 |
| Synonyms : |
|
Safety of [ 93413-76-4 ]
| Signal Word: | Warning |
Class: | N/A |
| Precautionary Statements: | P305+P351+P338 |
UN#: | N/A |
| Hazard Statements: | H315-H319 |
Packing Group: | N/A |
| GHS Pictogram: |

|
Application In Synthesis of [ 93413-76-4 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 93413-76-4 ]
- 1
-
isopropanolic HCl
[ No CAS ]

-
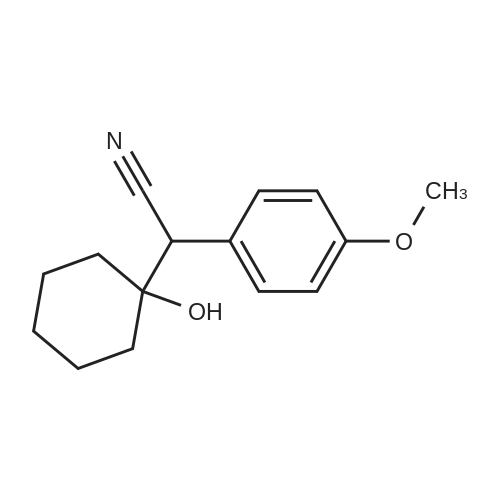 [ 93413-76-4 ]
[ 93413-76-4 ]

-
 [ 130198-05-9 ]
[ 130198-05-9 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With ammonium hydroxide;aluminum nickel; In methanol; ethyl acetate; |
b Hydrogenation A 5 liter autoclave is charged with 180 g of 1-[cyano(4-methoxyphenyl)methyl]cyclohexanol, 2400 ml of methanol, 600 ml of aqueous ammonia (25% by volume) and 135 g of Raney nickel (pretreated as given under 1a)) and the mixture is subjected to hydrogenation at 27 to 30 C. and 120 psi pressure of hydrogen for 9 to 10 hours. The reaction mixture is filtered through 100 g of the celite and the catalyst bed is washed with 700 ml of methanol. The filtrate is concentrated to get 167.1 g of the crude product as an oily residue. 1.1 g of the crude product is dissolved in 2 ml of dry ethyl acetate and about 2 ml of isopropanolic HCl are added (the pH of the solution is about 2). The solvent is removed at high vacuum to give 1.14 g of 1-[2-amino-1-(4-methoxyphenyl)ethyl]cyclohexanol hydrochloride. |
- 2
-
 [ 93413-76-4 ]
[ 93413-76-4 ]

-
 [ 130198-05-9 ]
[ 130198-05-9 ]
| Yield | Reaction Conditions | Operation in experiment |
| 91% |
With hydrogenchloride; hydrogen;palladium 10% on activated carbon; In methanol; ethanol; at 20℃; under 2625.26 Torr; for 6h;Product distribution / selectivity; |
Example 2; 0.98 g of (4 mmol) l-(l-cyano-l-(4-methoxyphenyl)methyl)cyclohexanol was dissolved in 15 ml of methanol. Then 2 ml of 2.5 M HCl in ethanol and 300 mg of 10% Pd/C were added and hydrogenation at ambient temperature for 6 h at 3.5 bar was performed. After the reaction was completed (HPLC area %: Product 98.3%, Starting compound 1.0%), the mixture was filtered and evaporated to give the title oily residue. 25 ml of diisopropyl ether was added followed by evaporation to approximately 3 ml. Additionaly, 4ml diisopropyl ether were admixed and stirred for 2 h on ice. After filtration 1.04 g of product (91%) was obtained. HPLC area %: Product 99.5 %. |
| 83% |
With hydrogenchloride; hydrogen;palladium 10% on activated carbon; In methanol; water; at 20℃; under 2625.26 Torr; for 6h;Product distribution / selectivity; |
Example 1; 0.98 g of (4 mmol) l-(l-cyano-l-(4-methoxyphenyl)methyl)cyclohexanol was dissolved in 15 ml <n="13"/>of methanol. Then 0.6 ml of HCl cone, and 300 mg of 10 % Pd/C were added and hydrogenation at ambient temperature for 6 h at 3.5 bar was performed. After the reaction was completed (HPLC area %: Product 97.32%, Starting compound 1.06%), the mixture was filtered and evaporated to give the title oily residue. Addition of 20 ml isopropanol and evaporation to approximate volume of 2 ml were followed. The mixture was stirred for 3 h at room temperature and 10 h at temperature below 0 C. The precipitated product was filtered off and yielded 0.95 g of product that corresponded to 83%. HPLC area %: Product 99.7 %. |
| 72% |
|
An autoclave was charged with methanol (200 ml), l-[Cyano-(4-methoxyphenyl) methyl]-cyclohexanol (II) (25 g, 0.1019 mole), palladium on charcoal (10%, 50% wet) (25 g), and hydrochloric acid (1-3 mole). While supplying hydrogen gas at 0-20 kg/cm2, the mixture was slowly heated to 4O0C and then heated to 40C-50C for about 7-12 hours EPO <DP n="22"/>with hydrogen pressure 15-20 kg/cm2. After completion of the reaction, the mass was cooled to 25C-30C and the hydrogen pressure was slowly released. The catalyst was filtered off and washed with methanol. The pH of the clear solution was adjusted to 10.5- 11.0 by the addition of a 50% solution of sodium hydroxide. The solution was then filtered through a celite bed. The solvent methanol was distilled out. The residue was extracted with ethyl acetate after diluting with water. The organic layer was washed with water, brine and the ethyl acetate lambdavas distilled out so as to leave behind 2 volumes of the ethyl acetate with the residue. Isopropanol hydrochloric acid (16%) (28 g) was added to the residue and the resulting mixture was stirred at 5C -1O0C for 40-60 minutes. The solid separated was filtered and washed with ethyl acetate to yield 18.2 g (72%) of l-[2-amino- l-(4-methoxyphenyl)ethyl]-cyclohexanol hydrochloride (III-a) with a purity of 99.9% area by HPLC. |
|
|
Example 2: Preparation of 1-(2-amino-1-[4-methoxyphenyl]ethyl)cyclohexanol hydrochloride 25 g of 1-(cyano-[4-methoxy phenyl]methylcyclohexanol was charged in 205 ml of dichloromethane at 25 C followed by 39.5 g of tetrabutylammonium borohydride, which was added in 10 min at 25 C. The solution was cooled to 0-5C followed by the slow addition of 29 g of methyl iodide in 30-45 min at 0-5 C. The temperature was maintained at 0-5C for 30 min., than slowly raised to 25-30 C and maintained for 90 min at 25-30C. Completion of the reaction was checked by thin layer chromatography. 20 ml of ethanol was slowly added at 25-30 C and stirred for 30 min. The pH was adjusted to 2.0 with hydrochloric acid (diluted) at 25-30 C. The product was extracted from dichloromethane using 2 x 125 ml of demineralised water. The aqueous layer was collected and the pH therof was readjusted to 10.5 to 11.0 with 48 % sodium hydroxide solution. Subsequently the product was extracted from the aqueous layer with 2 x 100 ml dichloromethane, the dichloromethane was collected and dried over sodium sulfate. After removing the sodium sulfate by filtration, hydrochloric acid gas was passed through the dichloromethane layer for 15-20 min at 25-30 C. The temperature was maintained at 25-30 C for 30 min. The dichloromethane was removed completely under reduced pressure at a temperature below 45 C, followed by the addition of 50 ml of acetone and cooling to 0-5 C. After stirring for 60 min. at 0-5C, the crystallized intermediate was filtered and washed with 25 ml of chilled (0-5 C) acetone. After drying of the powder for 60 min at 55-60 C the yield was 12.5 g. |
|
|
Example 3Step I: Preparation of l-[2-amino-l-(4-mcthoxy phenyl) ethyll cyclohexanol hydrochloride l-[Cyano-l-(4-methoxyphenyl)methyl]cyclohexanol (100.0 g) was dissolved in formic acid (800 ml; 98%) in an autoclave and cooled to 10-150C. Palladium catalyst (Pd/C 15%, 15.0 g: 50% wet) slurred in formic acid (45.0 ml) was added to the reaction mass and the reaction mass was stirred at 10 - 150C under hydrogen pressure 3.5 - 4.0 kg/cm2 for 4.0 hours. After complete conversion of l-[cyano-l-(4-methoxyphenyl) methyl] cyclohexanol, the reaction mass was filtered and formic acid was recovered under vacuum at 40 - 450C to obtain a viscous oily product. The oily product was dissolved in purified water (500 ml) and washed with ethyl acetate (3 x 100 ml). The aqueous layer was basified with 10% aqueous sodium hydroxide to pH 9.5 at 15 - 2O0C. The reaction mixture was extracted with ethyl acetate (750 ml x 3). The organic layer was combined and concentrated under vacuum at temperature of 45 - 5O0C. Ethyl acetate (450 ml) was added to the oil (115.0 g) with stirring and pH was adjusted with ethyl acetate-hydrochloric acid at 20 - 3O0C to pH 1 - 2. A thick white suspension was formed. The temperature of reaction mass was raised to 40 - 450C and stirred for 2.0 hours at this temperature, cooled to 10 - 150C and filtered. The wet product was slurred in ethyl acetate (200 ml) for 2.0 hours, filtered, washed with chilled ethyl acetate (100 ml) and dried under vacuum at 40 - 450C for 8 - 10 hours to obtain 70.5 g of title compound having purity 99.0% by high performance liquid chromatography.; Example 4Step I: Preparation of l-f2-amino-l-(4-methoxy phenyl) ethyllcyclohexanol hydrochloride l-[Cyano-l-(4-methoxyphenyl)methyl]cyclohexanol (100.0 g) was dissolved in formic acid (800 ml; 98%) in an autoclave and cooled to 10-150C. Palladium catalyst (Pd/C 15%, 15.0 g: 50% wet) slurred in formic acid (45.0 ml) was added to the reaction mass and the reaction mass was stirred at 10 - 150C under hydrogen pressure 3.5 - 4.0 kg/cm2 for 4.15 hours. After complete conversion of l-[cyano-l-(4-methoxyphenyl) methyl] cyclohexanol, the reaction mass was filtered and formic acid was recovered <n="12"/>under vacuum at 40 - 450C to obtain a viscous oily product. The oily product was dissolved in purified water (500 ml) and washed with ethyl acetate (3 x 100 ml). The aqueous layer was basified with 10% aqueous sodium hydroxide to pH 9.5 at 15 - 2O0C. The reaction mixture was extracted with ethyl acetate (750 ml x3). The organic layer was combined and concentrated under vacuum at temperature 45 - 5O0C. Ethyl acetate (450 ml) was added to the oil (115.0 g) with stirring and pH was adjusted with ethyl acetate-hydrochloric acid at 20 - 3O0C to pH 1 - 2. A thick white suspension was formed. The temperature of reaction mass was raised to 40 - 450C and stirred for 2.0 hours at this temperature, cooled to 10 - 150C and filtered. The wet product was slurred in ethyl acetate (200 ml) for 2.0 hours, filtered, washed with chilled ethyl acetate (100 ml) and dried under vacuum at 40 - 450C for 8 - 10 hours to obtain 65.2 g of title compound having purity 99.45 % by high performance liquid chromatography. |
|
|
EXAMPLE 1:; a) Preparation of 1 -[2-amino-l-(4-methoxy phenyl)ethyl]cyclohexanol hydrochloride (VII); 10 gm of p-methoxy phenyl acetonitrile, 6.85 gm of cyclohexanone, 25 ml of n-butanol were mixed slowly with stirring while maintaining the temperature in the range of - 10 to - 5 C for 15 to 20 mins. 20% solution of sodium butoxide was added to the reaction mixture slowly over a period of 25 to 30 minutes while maintaining the temperature in the range of -10 to - 5 0C. The reaction mixture was stirred for 120 minutes while maintaining the same temperature. The reaction mixture was acidified (pH about 5.5) with glacial acetic acid while maintaining the temperature in the range of -5 to 50C. 30 ml of water was added to the reaction mixture and was stirred for 10 minutes. The biphasic reaction mixture was allowed to settle and the organic layer was separated. To this organic layer, 12 gm Raney nickel catalyst and 10 % ammonical butanol (100 ml) were added. The reaction mixture was transferred to autoclave vessel. H2 gas was flushed to increase the pressure to 120psi. The mixture was slowly heated at temperature of 10 to 120C under the hydrogen pressure of 120 psi for 6 to 7 hrs. The completion of the reaction was monitored with thin layer chromatography. After completion of reaction, the catalyst was filtered off and the solvent was distilled off to obtain residue. The residue was dissolved in 60 ml ethyl acetate and cooled to 0-50C. 20 % solution of hydrogen chloride in isopropyl alcohol was added to the solution with stirring to adjust the pH to 1-1.5. The reaction mixture was further stirred for 30 to 35 minutes. 7.5 ml of n-hexane was added to the solution with stirring. Further the reaction mixture was stirred for 3 hours at 0 to 50C. Precipitated l-[2-amino-l-(4-methoxy phenyl)ethyl]cyclohexanol hydrochloride (VII) was filtered and dried at 50 to 520C. % Yield : 90 % % Purity : 95 % |
|
|
EXAMPLE 2:; a) Preparation of l-[2-amino-l-(4-methoxy phenyl)ethyl]cyclohexanol hydrochloride (VII); 10 gm of p-methoxy phenyl acetonitrile, 6.85 gm of cyclohexanone, 25 ml of isobutanol were mixed slowly with stirring while maintaining the temperature in the range of -10 to -5 C for 15 to 20 mins. 20% solution of sodium isobutoxide was added to the reaction mixture slowly over a period of 25 to 30 minutes while maintaining the temperature in the range of -10 to -50C. The reaction mixture was stirred for 120 minutes while maintaining the same temperature. The reaction mixture was acidified (pH about 5.5) with glacial acetic acid while maintaining the temperature in the range of -5 to 5C. 30 ml of water was added to the reaction mixture and was stirred for 10 minutes. The biphasic reaction mixture was allowed to settle and the organic layer was separated. To this organic layer, 12 gm Raney nickel catalyst and 10 % ammonical isobutanol (100 ml) were added. The reaction mixture was transferred to autoclave vessel. H2 gas was flushed to increase the pressure to 120psi. The mixture was slowly heated at temperature of 10 to 120C under the hydrogen pressure of 120 psi for 6 to 7 hrs. The completion of the reaction was monitored with thin layer chromatography. After completion of reaction, <n="14"/>the catalyst was filtered off and the solvent was distilled off to obtain residue. The residue was dissolved in 60 ml ethyl acetate and cooled to 0-50C. 20 % solution of hydrogen chloride in isopropyl alcohol was added to the solution with stirring to adjust the pH to 1-1.5. The reaction mixture was further stirred for 30 to 35 minutes. 7.5 ml of n-hexane was added to the solution with stirring. Further the reaction mixture was stirred for 3 hours at 0 to 50C. Precipitated l-[2-amino-l-(4-methoxy phenyl)ethyl]cyclohexanol hydrochloride (VII) was filtered and dried at 50 to 520C. % Yield : 89.1 % % Purity : 94.5 % |
|
|
EXAMPLE 3:; a) Preparation of 1 - [2-amino- 1 -(4-methoxy phenyl)ethyl] cyclohexanol hydrochloride (VII); 10 gm of p-methoxy phenyl acetonitrile, 6.8 gm of cyclohexanone, 25 ml of tert-butanol were mixed slowly with stirring while maintaining the temperature in the range of -10 to -5 C for 15 to 20 mins. 20% solution of sodium tert-butoxide was added to the reaction mixture slowly over a period of 25 to 30 minutes while maintaining the temperature in <n="15"/>the range of -10 to -5 0C. The reaction mixture is stirred for 120 minutes while maintaining the same temperature. The reaction mixture was acidified (pH about 5.5) with glacial acetic acid while maintaining the temperature in the range of -5 to 50C. 30 ml of water was added to the reaction mixture and was stirred for 10 minutes. The biphasic reaction mixture was allowed to settle and the organic layer was separated. To this organic layer, 12 gm Raney nickel catalyst and 10 % ammonical tert-butanol (100 ml) were added. The reaction mixture was transferred to autoclave vessel. H2 gas was flushed to increase the pressure to 120psi. The mixture was slowly heated at temperature of 10 to 120C under the hydrogen pressure of 120 psi for 6 to 7 hrs. The completion of the reaction was monitored with thin layer chromatography. After completion of reaction, the catalyst was filtered off and the solvent was distilled off to obtain residue. The residue was dissolved in 60 ml ethyl acetate and cooled to 0-50C. 20 % solution of hydrogen chloride in isopropyl alcohol was added to the solution with stirring to adjust the pH to 1-1.5. The reaction mixture was further stirred for 30 to 35 minutes. 7.5 ml of n-hexane was added to the solution with stirring. Further the reaction mixture was stirred for 3 hours at 0 to 50C. Precipitated l-[2-amino-l-(4-methoxy phenyl)ethyl]cyclohexanol hydrochloride (VII) was filtered and dried at 50 to 520C. % Yield : 88.5 % % Purity : 94.1 % |

Reference:
[1]ChemistryOpen,2017,vol. 6,p. 211 - 215
[2]Patent: WO2007/147564,2007,A1 .Location in patent: Page/Page column 12
[3]Patent: WO2007/147564,2007,A1 .Location in patent: Page/Page column 11-12
[4]Patent: WO2007/47972,2007,A2 .Location in patent: Page/Page column 20; 21
[5]Patent: EP1721889,2006,A1 .Location in patent: Page/Page column 5-6
[6]Patent: WO2007/49302,2007,A2 .Location in patent: Page/Page column 9
[7]Patent: WO2007/69277,2007,A2 .Location in patent: Page/Page column 11
[8]Patent: WO2007/69277,2007,A2 .Location in patent: Page/Page column 12-13
[9]Patent: WO2007/69277,2007,A2 .Location in patent: Page/Page column 13-14
[10]Patent: US2015/299102,2015,A1

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping