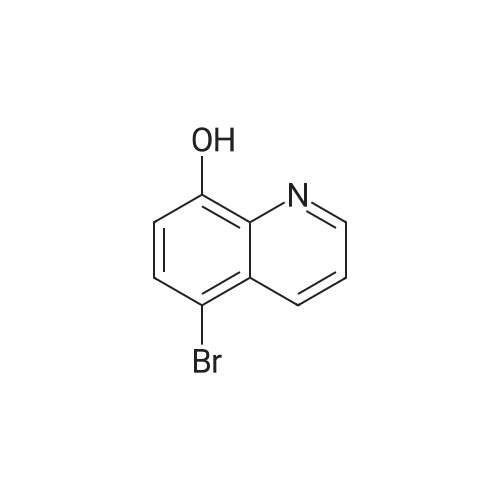
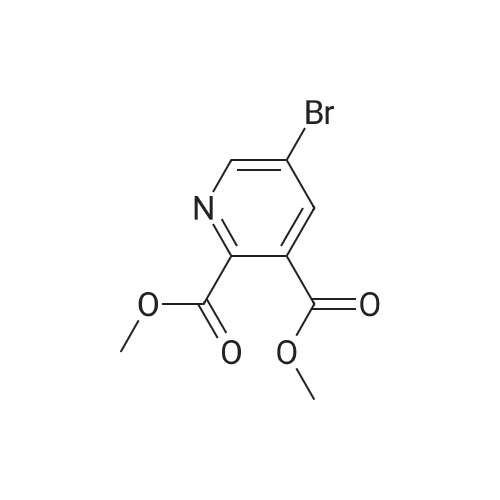
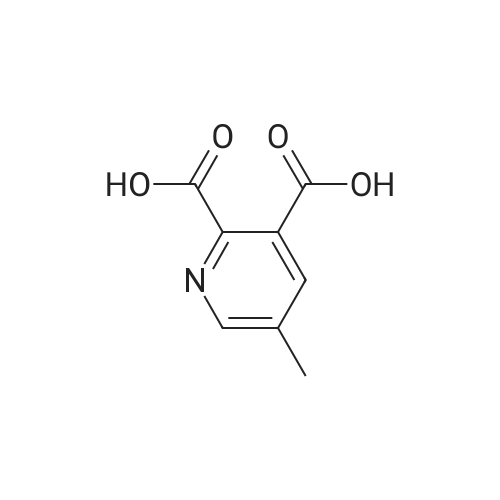
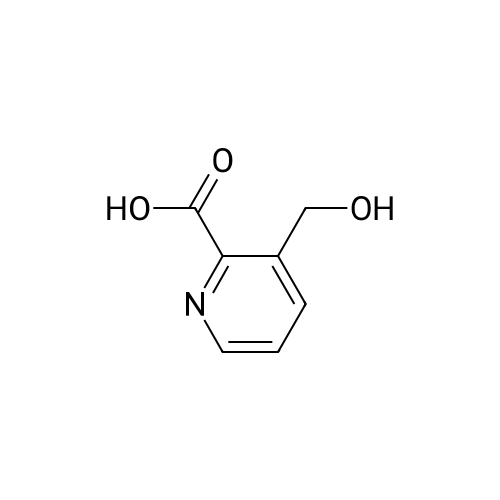
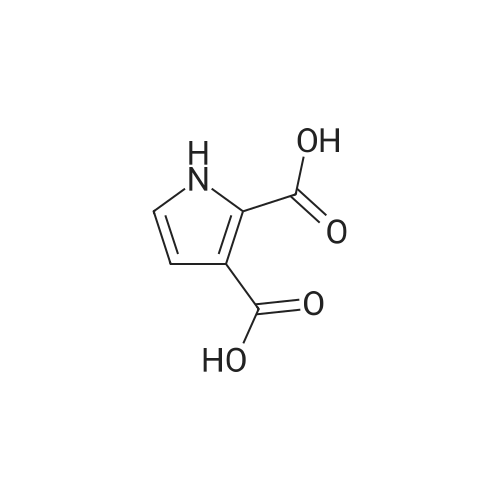
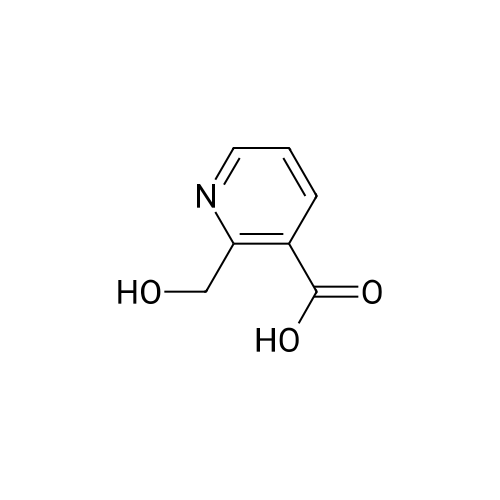
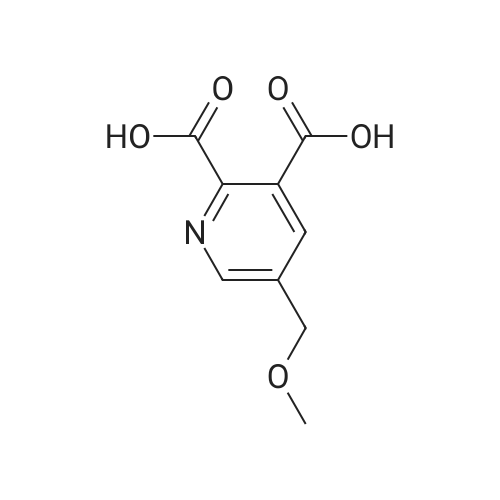
| 63% |
|
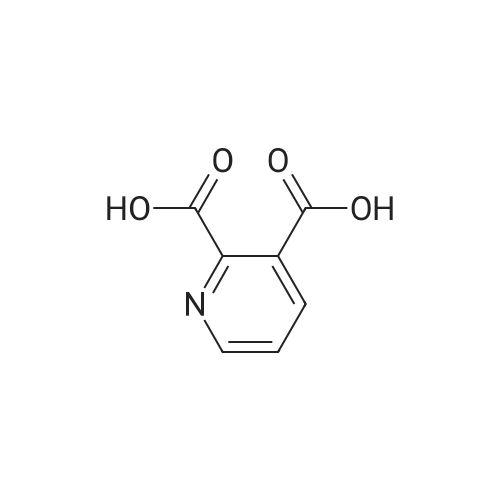
To a suspension of pyridine-2,3-dicarboxylic acid (50 g, 300 mmol) in MeOH (500 mL) was added concentrated H2SO4 (10 mL) slowly. The mixture was heated at reflux for 48 h, then cooled to 40 C, followed by the dropwise addition of Br2 (38 mL, 750 mmol) over 2 h. The reaction was heated at 55 C for 48 h, and then concentrated in vacuo. The residue was dissolved in isopropyl acetate (500 mL), and the resulted solution was washed with saturated aqueous a2S203 (200 mL x 4), followed by brine (400 mL), dried over anhydrous Na2S04, filtered and concentrated in vacuo. The residue was purified by a silica gel column chromatography (PE/EtOAc (v/v) = 5/1) to give the title compound as a yellow solid (52 g, 63%). MS (ESI, pos. ion) m/z: 274.0 [M + H]+; FontWeight="Bold" FontSize="10" H NMR (400 MHz, CDC13) delta (ppm): 8.75-8.76 (d, J= 2.2 Hz, 1H), 8.23-8.24 (d, J= 2.2 Hz, 1H), 3.93 (s, 1H), 3.89 (s, 1H). |
| 63% |
|
A solution of pyridyl 2,3-dicarboxylic acid (50 g, 300 mmol)Suspended in methanol (500 mL)To this was slowly added concentrated sulfuric acid (10 mL).After the mixture was refluxed for 48 hours,Cooled to 40 C, within 2 hours,To this was added bromine (38 mL, 750 mmol).The reaction solution was stirred at 55 C for 48 hours,Concentrated under reduced pressure.The residue was dissolved in isopropyl acetate (500 mL) and the resulting solution was obtainedThe residue was washed with sodium thiosulfate saturated solution (200 mL x 4), brine (400 mL), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure.The residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate (v / v) = 5/1) to give the title compound as a yellow solid (52 g, 63%) |
|
|
Method II:; Step A: Dimethyl 5-bromopyridine-2,3-dicarboxylate; Concentrated sulfuric acid (1 L, 18.7 mol) was added slowly over 10 min to a suspension of pyridine-2,3-dicarboxylic acid (5.00 kg, 29.9 mol) in methanol (50 L), dissolving the suspension. The resulting mixture was heated at reflux for 48 h then cooled to 40 C. Bromine (8.0 kg, 50 mol) was added slowly over 2 h in 1-kg portions, keeping the temperature below 55 C. The reaction mixture was then heated at 55 C. for 24 h, cooled to 50 C. and additional Br2 (4.0 kg, 25 mol) was added slowly over 1 h in 1-kg portions, keeping temperature below 55 C. The reaction mixture was heated at 55 C. for 24 h, concentrated to a minimum volume (internal temp 30 C., solution may occasionally foam), then diluted with isopropyl acetate (50 L) and washed with a saturated aqueous sodium sulfite solution (3×20 L) (final extract is pH 8) followed by water (20 L). The organic layer was concentrated to approximately 15 L then diluted with heptane (40 L). The resulting slurry was stirred for 24 h at 23 C. The solids were filtered, washed with heptane (10 L), and dried to give the title compound. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping