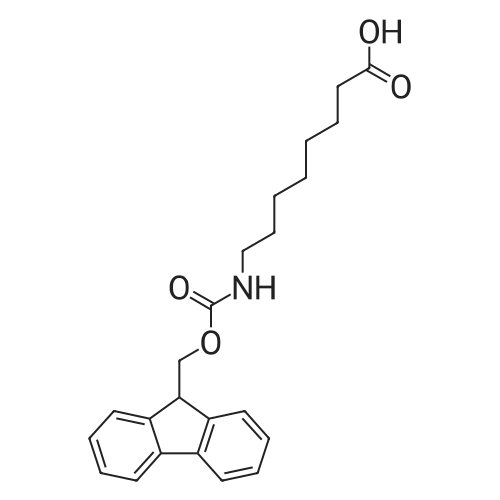
Alternatived Products of [ 88574-06-5 ]
Product Details of [ 88574-06-5 ]
| CAS No. : | 88574-06-5 |
MDL No. : | MFCD00077045 |
| Formula : |
C21H23NO4
|
Boiling Point : |
- |
| Linear Structure Formula : | C6H12O2NHC15H10O2 |
InChI Key : | FPCPONSZWYDXRD-UHFFFAOYSA-N |
| M.W : |
353.41
|
Pubchem ID : | 2756087 |
| Synonyms : |
|
Chemical Name : | Fmoc-ε-Acp-OH |
Application In Synthesis of [ 88574-06-5 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 88574-06-5 ]
- 1
-
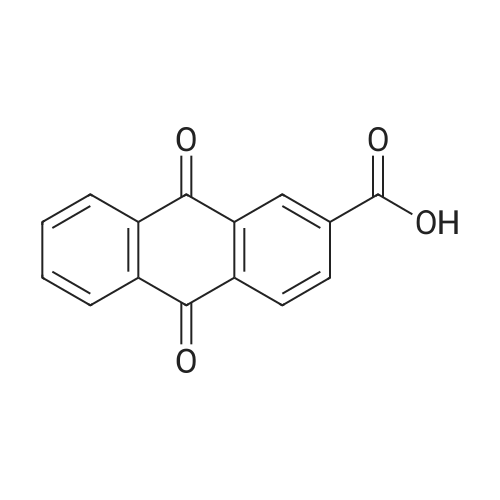 [ 117-78-2 ]
[ 117-78-2 ]

-
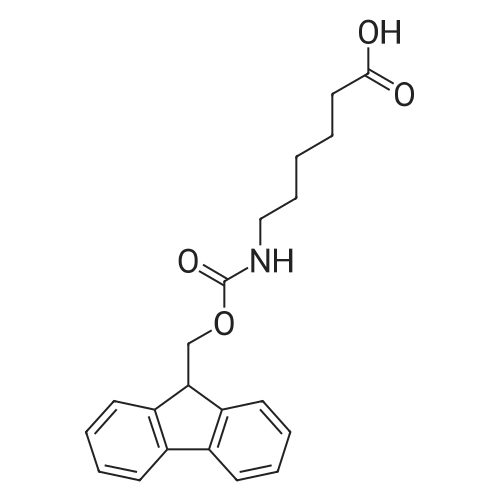 [ 88574-06-5 ]
[ 88574-06-5 ]

-
ArgPmc-Ual-Sar-Chi-Chi-Tal-ArgPmc-Rink amide MBHA resin
[ No CAS ]

-
anthraquinone-2-carbonyl-6-NH-hexanoyl-Arg-β-(uracyl-1-yl)-D-alanyl-Sar-[β-(1,2,3,4-tetrahydro-2,4-dioxoquinazolin-3-yl)-D-alanyl]2-β-(thymin-1-yl)-D-alanyl-Arg-NH2
[ No CAS ]
- 2
-
coenzyme A trilithium salt
[ No CAS ]

-
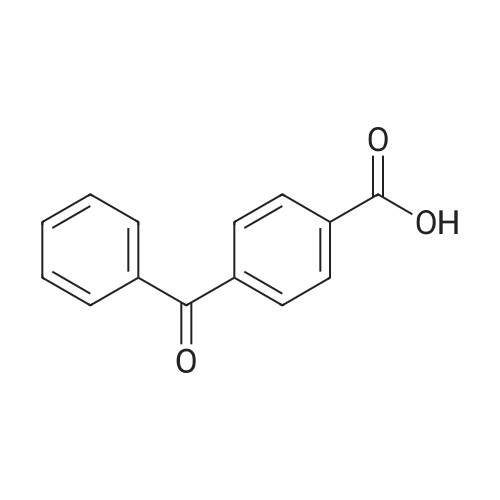 [ 611-95-0 ]
[ 611-95-0 ]

-
QTARKSTGGK(1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)-3-methylbutyl)APRKQLATK
[ No CAS ]

-
 [ 79-08-3 ]
[ 79-08-3 ]

-
 [ 88574-06-5 ]
[ 88574-06-5 ]

-
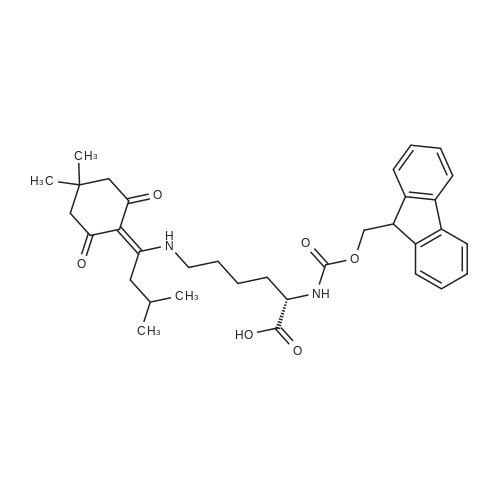 [ 204777-78-6 ]
[ 204777-78-6 ]

-
 [ 198561-07-8 ]
[ 198561-07-8 ]

-
histone QTARKSTGGK(1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)-3-methylbutyl)PRKQLATK-lysine 14-coenzyme A-benzophenone-L-propargylglycine
[ No CAS ]
- 3
-
coenzyme A trilithium salt
[ No CAS ]

-
 [ 611-95-0 ]
[ 611-95-0 ]

-
SGRGKGGKGLGKGGAK(1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)-3-methylbutyl)RHR
[ No CAS ]

-
 [ 79-08-3 ]
[ 79-08-3 ]

-
 [ 88574-06-5 ]
[ 88574-06-5 ]

-
 [ 204777-78-6 ]
[ 204777-78-6 ]

-
 [ 198561-07-8 ]
[ 198561-07-8 ]

-
histone SGRGKGGKGLGKGGAK(1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)-3-methylbutyl)RHR-lysine 16-coenzyme A-benzophenone-L-propargylglycine
[ No CAS ]
- 4
-
coenzyme A trilithium salt
[ No CAS ]

-
 [ 611-95-0 ]
[ 611-95-0 ]

-
 [ 79-08-3 ]
[ 79-08-3 ]

-
 [ 88574-06-5 ]
[ 88574-06-5 ]

-
 [ 204777-78-6 ]
[ 204777-78-6 ]

-
 [ 198561-07-8 ]
[ 198561-07-8 ]

-
 [ 1612162-71-6 ]
[ 1612162-71-6 ]
- 5
-
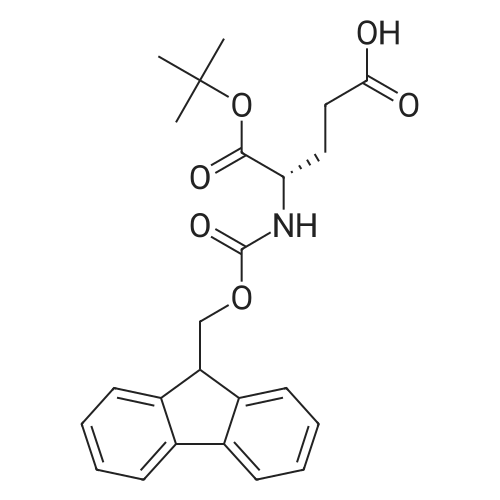 [ 84793-07-7 ]
[ 84793-07-7 ]

-
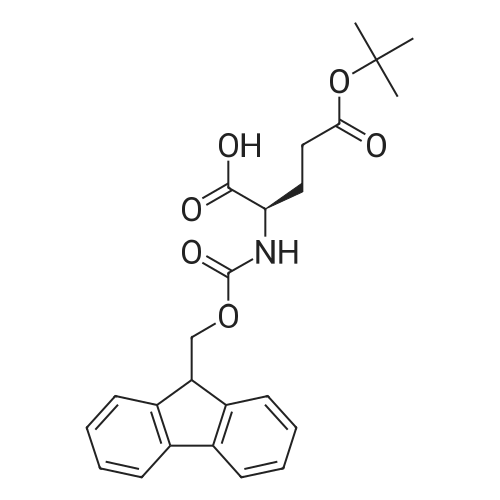 [ 104091-08-9 ]
[ 104091-08-9 ]

-
 [ 88574-06-5 ]
[ 88574-06-5 ]

-
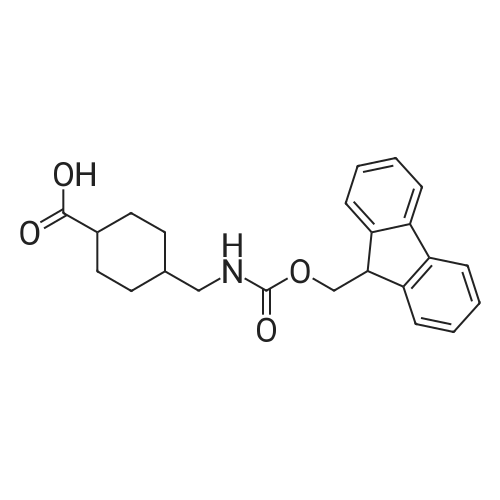 [ 188715-40-4 ]
[ 188715-40-4 ]

-
(S)-6-[(Diphenyl-p-tolyl-methyl)-amino]-2-(9H-fluoren-9-ylmethoxycarbonylamino)-hexanoic acid
[ No CAS ]

-
C46H74N10O20
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
PSMA-i was synthesized manually using standard Fmoc chemistry. Generally, peptide was synthesized at 0.2 mmol scale starting from C-terminal Fmoc-rink amide MBHA resin. Fmoc-deprotection at each cycle was carried out using 20% pipperidine in DMF. Coupling reactions were caffied out using 3.3 equiv of Fmoc-amino acids in DMF activated with 3.3 equiv of HCTU and 5 equiv of diisopropylethylamine (DIPEA) in DMF. These steps were repeated each time with an amino acid added. After the peptide sequence Fmoc-Glu?-Amc-Ahx-Glu-Glu-Glu-Lys(Mtt) was built on the resin, the Fmoc group of Nterminal amino acid Glu? was deprotected by 20% pipperidine. Then, a chloroform solution containing 3 eq of H-Glu(OtBu)-OtBu mixed with 2.5eq of DIPEA were prepared. The solution is then added slowly to 0.25 eq triphosgene in chloroform over 10 minutes at room temperature. After 15 minute incubation to allow for isocyanate formation, the reaction mixture was mixed with Glu?-Amc-Ahx-Glu-Glu-Glu-Lys on rink amide resin pre-swollen in chloroform with 2.5 eq of DIPEA. After the reaction was complete, the resin was washed with DMF and then dichloromethane and dried. The peptide was cleaved from resin by TFA/water/triisopropylsilane (950:25:25). The cleaved peptide was purified by preparative HPLC. The products were ascertained by high resolution matrix-assisted laser desorption/ionization mass (MALDI-MS) spectra from an Applied Biosystem 4800 MALDI TOF/TOF Analyzer in positive ion mode. Retention time: 19.0 mm. MALDI MS:C48H74N10020, 1087.5 (found); 1087.1 (calculated). |
- 6
-
 [ 404858-60-2 ]
[ 404858-60-2 ]

-
Paramax Wang resin
[ No CAS ]

-
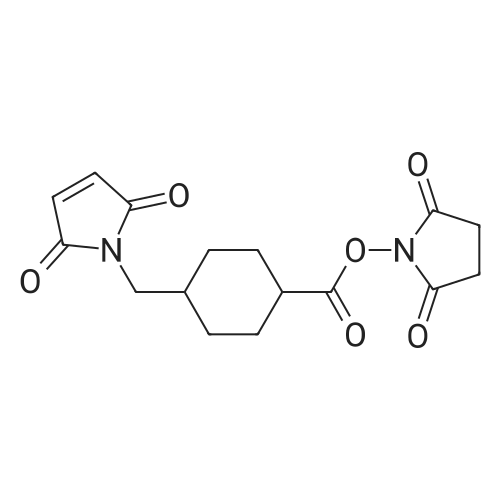 [ 64987-85-5 ]
[ 64987-85-5 ]

-
 [ 29022-11-5 ]
[ 29022-11-5 ]

-
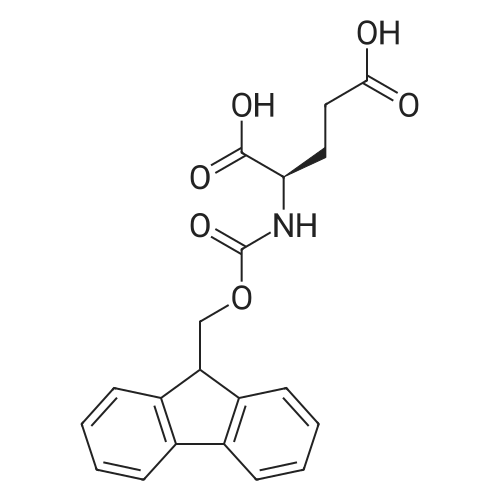 [ 104091-09-0 ]
[ 104091-09-0 ]

-
 [ 88574-06-5 ]
[ 88574-06-5 ]

-
C44H64N8O22S
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
Synthesis of Compound 1 (ZJ-MCC-Ahx-dEdEdEG): The peptide Fmoc-Ahx-dGlu-dGlu-dGlu-G was assembled on a Wang resin. The three glutamates (dGlu) are of D-isoform. Peptide synthesis was carried out manually by Fmoc chemistry with HCTU (2-(6-Chloro-1H-benzotriazole-1-yl)-1,1,3,3-tetramethylaminium hexafluorophosphate) activation. Generally, peptides were synthesized at a 0.01 mmol scale starting from the C-terminal amino acid on solid support. Fmoc-deprotection at each cycle was carried out using 20percent piperidine in DMF. Coupling reactions were carried out using 3.3 eq. of Fmoc-amino acids in DMF activated with 3.3 eq. of HCTU and 5 equivalents of diisopropylethylamine (DIPEA) in DMF. These steps were repeated each time with an amino acid added. After the peptide sequence was built on the resin, the Fmoc group of the N-terminal amino acid was deprotected. Coupling of 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) to the N-terminal amine group was achieved with 3.3 equivalents of SMCC in DMF. Coupling of Cys-C(O)-Glu was performed using 3.3 equivalents of Cys-C(O)-Glu in DMF after coupling SMCC to the peptide. The final peptide resin was washed with DMF and then dichloromethane and dried. Cleavage and deprotection were carried out using TFA/water/triisopropylsilane (950:25:25) for 1 h, the resin was removed by filtration and washed with TFA. The combined filtrate was dried under nitrogen. The synthesized peptide was precipitated by the addition of diethyl ether and collected by centrifugation. The cleaved peptide was purified by preparative HPLC. The products were ascertained by high resolution matrix-assisted laser desorption/ionization mass (MALDI-MS) spectra. Then Fmoc was deprotected followed by coupling of SMCC and Cys-C(O)-Glu. The product has retention time of 11.9 minutes on analytical HPLC with 0-55percent gradient over 45 minutes (flow rate 1 ml/min; A: 10 mM triethylammonium acetate TEAA, pH 7.0; B was acetonitrile.) The mass was verified by MALDI/TOF mass spectrometry?Calculated: 1088.4 (C44H64N8O22S). Found m/z: 1089.4 (M+1). |
- 7
-
C22H39N6O9Pol
[ No CAS ]

-
Fmoc‐D‐Cys(Trt)‐OPfp
[ No CAS ]

-
 [ 35661-40-6 ]
[ 35661-40-6 ]

-
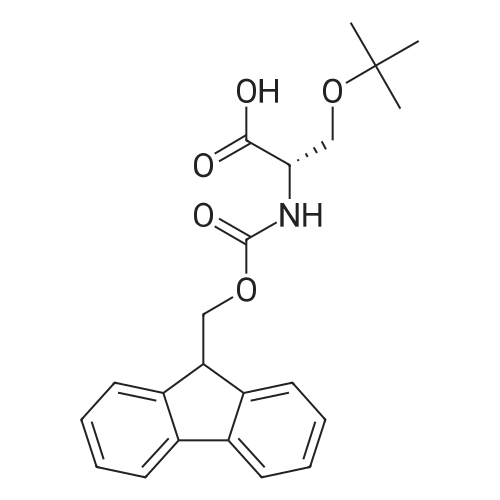 [ 71989-33-8 ]
[ 71989-33-8 ]

-
 [ 71989-38-3 ]
[ 71989-38-3 ]

-
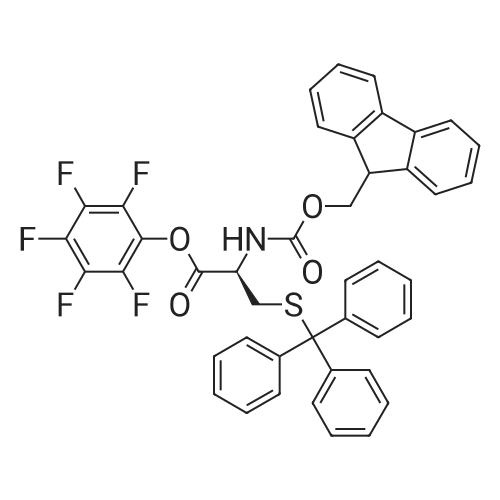 [ 115520-21-3 ]
[ 115520-21-3 ]

-
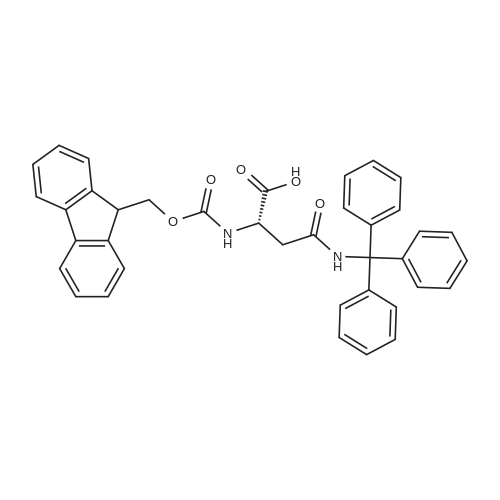 [ 132388-59-1 ]
[ 132388-59-1 ]

-
 [ 71989-23-6 ]
[ 71989-23-6 ]

-
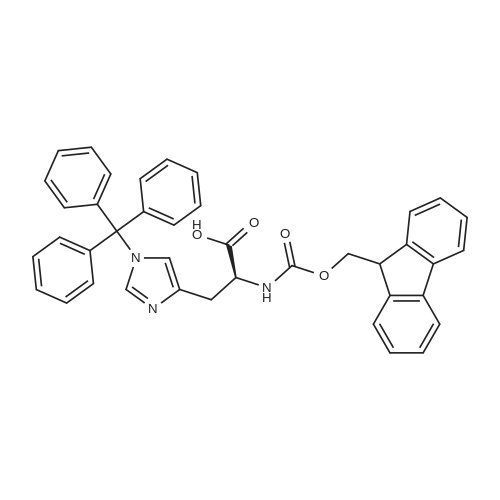 [ 109425-51-6 ]
[ 109425-51-6 ]

-
 [ 88574-06-5 ]
[ 88574-06-5 ]

-
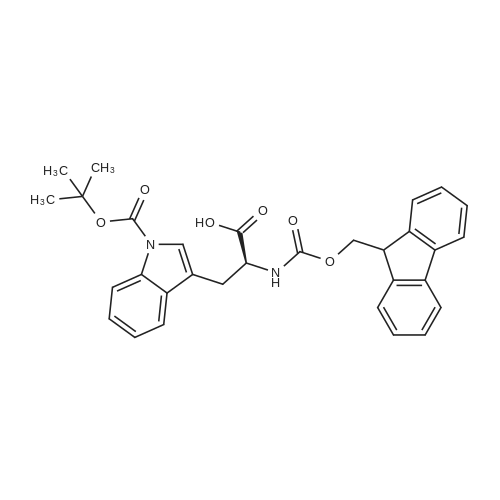 [ 143824-78-6 ]
[ 143824-78-6 ]

-
 [ 204777-78-6 ]
[ 204777-78-6 ]

-
C96H142N26O27S2
[ No CAS ]
- 8
-
 [ 29022-11-5 ]
[ 29022-11-5 ]

-
C22H39N6O9Pol
[ No CAS ]

-
Fmoc‐D‐Cys(Trt)‐OPfp
[ No CAS ]

-
 [ 35661-40-6 ]
[ 35661-40-6 ]

-
 [ 71989-33-8 ]
[ 71989-33-8 ]

-
 [ 71989-38-3 ]
[ 71989-38-3 ]

-
 [ 115520-21-3 ]
[ 115520-21-3 ]

-
 [ 132388-59-1 ]
[ 132388-59-1 ]

-
 [ 71989-23-6 ]
[ 71989-23-6 ]

-
 [ 88574-06-5 ]
[ 88574-06-5 ]

-
 [ 143824-78-6 ]
[ 143824-78-6 ]

-
 [ 204777-78-6 ]
[ 204777-78-6 ]

-
C90H135N23O26S2
[ No CAS ]
- 9
-
 [ 88574-06-5 ]
[ 88574-06-5 ]

-
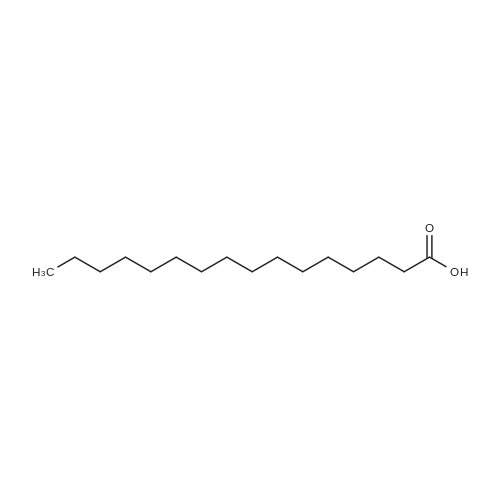 [ 57-10-3 ]
[ 57-10-3 ]

-
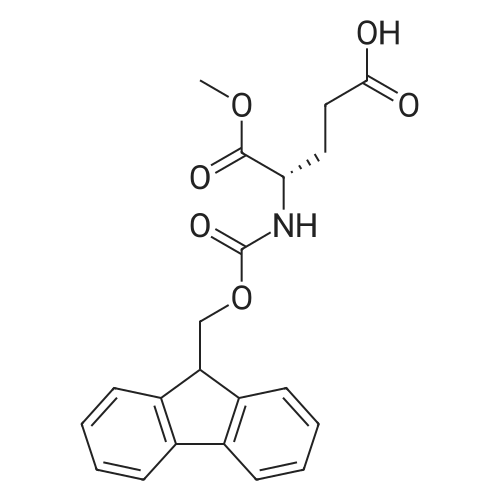 [ 145038-49-9 ]
[ 145038-49-9 ]

-
C28H52N2O6
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 68% |
|
Molecule A27 is obtained by means of the conventional solid phase peptide synthesis (SPPS) method on 2-chlorotrityl chloride (CTC) resin (24.00 g, 1.37 mmol/g). (1288) The grafting of the first amino acid Fmoc-6-aminohexanoic acid (1.5 equivalents) is performed in DCM (10 V), in the presence of DIPEA (2.5 equivalents). The unreacted sites are capped with methanol (0.8 mL/g resin) at the end of the reaction. (1289) The protected amino acid <strong>[145038-49-9]Fmoc-Glu-OMe</strong> (1.5 equivalents) and palmitic acid (1.5 equivalents) are coupled in DMF (10 V), in the presence of HATU (1.0 equivalent with respect to the acid) and DIPEA (1.5 equivalents with respect to the acid). (1290) The protecting groups Fmoc are removed using an 80:20 DMF/piperidine solution (10 V). (1291) The product is cleaved from the resin using an 80:20 DCM/HFIP solution (10 V). (1292) After concentration at reduced pressure, two co-evaporations are performed on the residue with dichloromethane followed by toluene. The product is purified by recrystallization in ethyl acetate. A white solid of molecule A27 is obtained. (1293) Yield: 11.54 g (68% in 6 stages) (1294) 1H NMR (CDCl3, ppm): 0.88 (3H); 1.19-1.35 (24H); 1.35-1.44 (2H); 1.50-1.70 (6H); 1.91-2.01 (1H); 2.14-2.40 (7H); 3.14-3.34 (2H); 3.75 (3H); 4.51-4.59 (1H); 6.53 (1H); 6.70 (1H). (1295) LC/MS (ESI+): 513.4 (calculated ([M+H]+): 513.4). |
- 10
-
 [ 88574-06-5 ]
[ 88574-06-5 ]

-
 [ 57-10-3 ]
[ 57-10-3 ]

-
 [ 145038-49-9 ]
[ 145038-49-9 ]

-
C28H52N2O6
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 11.54 g |
|
Molecule A27 is obtained by means of the conventional solid phase peptide synthesis (SPPS) method on 2-chlorotrityl chloride (CTC) resin (24.00 g, 1.37 mmol/g). The grafting of the first amino acid Fmoc-6-aminohexanoic acid (1.5 equivalents) is performed in DCM (10 V), in the presence of DIPEA (2.5 equivalents). The unreacted sites are capped with methanol (0.8 mL/g resin) at the end of the reaction. The protected amino acid <strong>[145038-49-9]Fmoc-Glu-OMe</strong> (1.5 equivalents) and palmitic acid (1.5 equivalents) are coupled in DMF (10 V), in the presence of HATU (1.0 equivalent with respect to the acid) and DIPEA (1.5 equivalents with respect to the acid). The protecting groups Fmoc are removed using an 80:20 DMF/piperidine solution (10 V). The product is cleaved from the resin using an 80:20 DCM/HFIP solution (10 V). After concentration under reduced pressure, two co-evaporations are performed on the residue with dichloromethane followed by toluene. The product is purified by recrystallization in ethyl acetate. A white solid of molecule A27 is obtained. Yield: 11.54 g (68% in 6 stages) 1H NMR (CDCl3, ppm): 0.88 (3H); 1.19-1.35 (24H); 1.35-1.44 (2H); 1.50-1.70 (6H); 1.91-2.01 (1H); 2.14-2.40 (7H); 3.14-3.34 (2H); 3.75 (3H); 4.51-4.59 (1H); 6.53 (1H); 6.70 (1H). LC/MS (ESI+): 513.4 (calculated ([M+H]+): 513.4). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping