| 86% |
With caesium carbonate;[1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II); In water; dimethyl sulfoxide; at 45℃; |
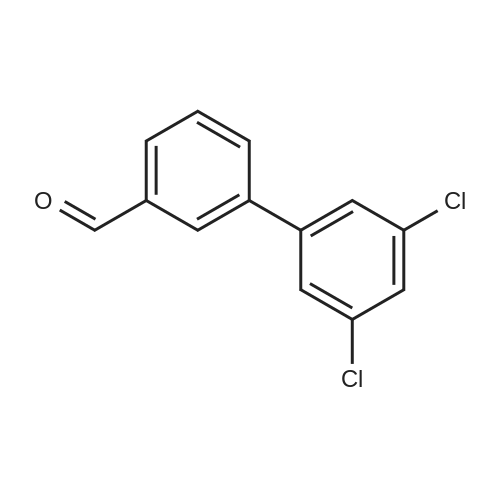
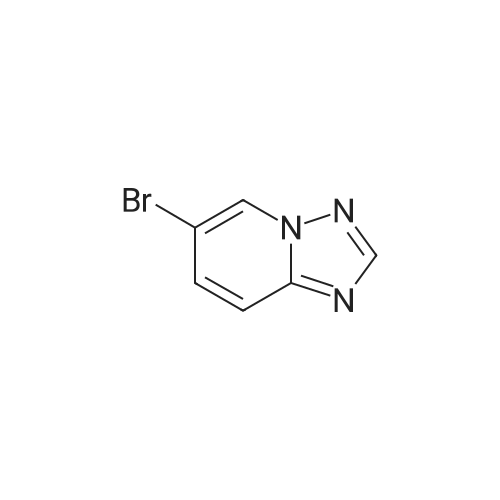
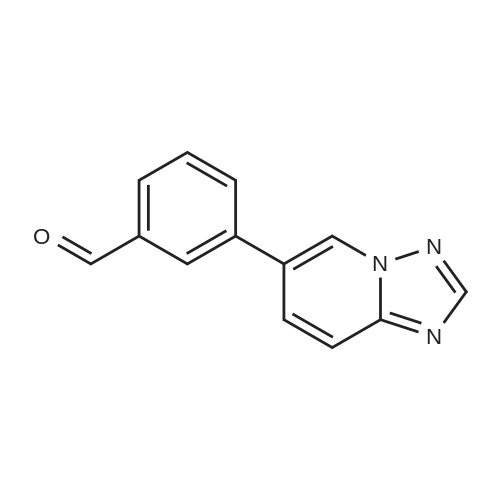
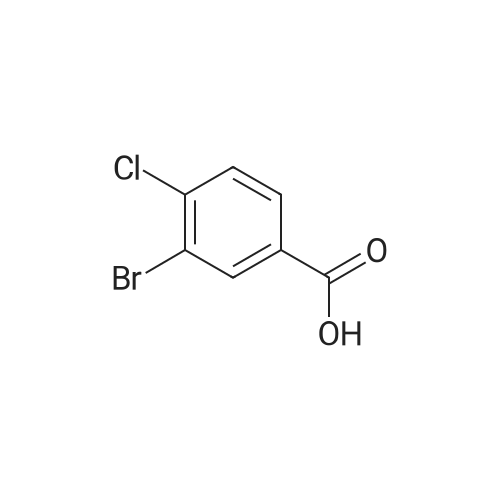
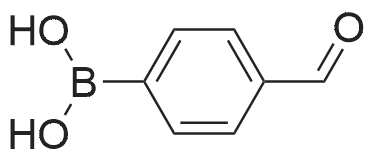
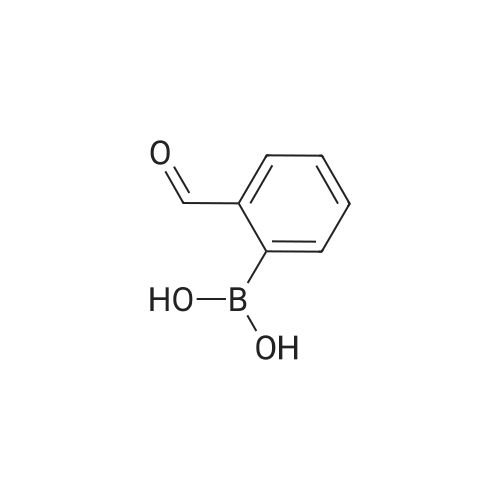
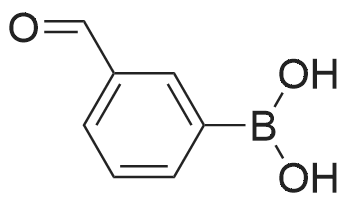
Step C: To a mixture of 3-formylphenylboronic acid (21.41 g, 143 mmol),6-bromo-[l,2,4]triazolo[l,5-a]pyridine (28.27 g, 143 mmol) in DMSO (600 mL) and water (50 mL) was added Pd(dppf)Cl2 (5.83 g, 7.14 mmol) and Cs2CO3 (116 g, 357 mmol). The reaction temperature reached 45 0C after the addition. HPLC showed that starting materials were consumed after 15 min. The reaction was diluted with water (400 mL). The black precipitate was collected by filtration and dissolved in DCM (300 mL), and washed with brine (200 mL). The aqueous layer was back extracted with DCM (100 mL). The combined organic layers were filtered through a Celite pad and the filtrate was concentrated to give a black solid mixture. The product was recrystallized in methanol to give 3-([l,2,4]triazolo[l,5-a]pyridin-6-yl)benzaldehyde (27.4 g, 123 mmol, 86 % yield) as a pale grey solid: m/z = 224.0 [M+l]; 1H NMR (400 MHz, DMSO-D6) delta ppm 7.74 (t, J=7.68 Hz, 1 H), 7.91 - 8.02 (m, 2 H), 8.11 (dd, J=9.19, 1.89 Hz, 1 H), 8.17 (d, J=7.81 Hz, 1 H), 8.36 (s, 1 H), 8.57 (s, 1 H), 9.45 (s, 1 H), 10.11 (s, 1 H). |
| 86% |
With caesium carbonate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In water; dimethyl sulfoxide; at 45℃; for 0.25h; |
To a mixture of 3-formylphenylboronic acid (21.41 g, 143 mmol),6-bromo-[l,2,4]triazolo[l,5-a]pyridine (28.27 g, 143 mmol) in DMSO (600 mL) and water (50 mL) was added Pd(dppf)Cl2 (5.83 g, 7.14 mmol) and Cs2CO3 (116 g, 357 mmol). The reaction temperature reached 45 0C after the addition. HPLC showed that starting materials were consumed after 15 min. The reaction was diluted with water (400 mL). The black precipitate was collected by filtration and dissolved in DCM (300 mL), and washed with brine (200 mL). The aqueous layer was back extracted with DCM (100 mL). The combined organic layers were filtered through a Celite pad and the filtrate was concentrated to give a black solid mixture. The product was recrystallized in methanol to give 3-([l,2,4]triazolo[l,5-a]pyridin-6-yl)benzaldehyde (27.4 g, 123 mmol, 86 % yield) as a pale grey solid: m/z = 224.0 [M+l]; 1H NMR (400 MHz, DMSO-D6) delta ppm 7.74 (t, J=7.68 Hz, 1 H), 7.91 - 8.02 (m, 2 H), 8.11 (dd, J=9.19, 1.89 Hz, 1 H), 8.17 (d, J=7.81 Hz, 1 H), 8.36 (s, 1 H), 8.57 (s, 1 H), 9.45 (s, 1 H), 10.11 (s, 1 H). |
| 86% |
With caesium carbonate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In water; dimethyl sulfoxide; at 45℃; for 0.25h; |
To a mixture of 3-formylphenylboronic acid (21.41 g, 143 mmol),6-bromo-[l,2,4]triazolo[l,5-a]pyridine (28.27 g, 143 mmol) in DMSO (600 mL) and water (50 mL) was added Pd(dppf)Cl2 (5.83 g, 7.14 mmol) and Cs2CO3 (116 g, 357 mmol). The reaction temperature reached 45 0C after the addition. HPLC showed that starting materials were consumed after 15 min. The reaction was diluted with water (400 mL). The black precipitate was collected by filtration and dissolved in DCM (300 mL), and washed with brine (200 mL). The aqueous layer was back extracted with DCM (100 mL). The combined organic layers were filtered through a Celite pad and the filtrate was concentrated to give a black solid mixture. The product was recrystallized in methanol to give 3-([l,2,4]triazolo[l,5-a]pyridin-6-yl)benzaldehyde (27.4 g, 123 mmol, 86 % yield) as a pale grey solid: m/z = 224.0 [M+l]; 1H NMR (400 MHz, DMSO-D6) delta ppm 7.74 (t, 7=7.68 Hz, 1 H), 7.91 - 8.02 (m, 2 H), 8.11 (dd, 7=9.19, 1.89 Hz, 1 H), 8.17 (d, 7=7.81 Hz, 1 H), 8.36 (s, 1 H), 8.57 (s, 1 H), 9.45 (s, 1 H), 10.11 (s, 1 H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping