Mg2+-Ion Dependence Revealed for a BAHD 13-O-β-Aminoacyltransferase from Taxus Plants
Al-Hilfi, Aimen
;
Li, Zhen
;
Merz Jr, Kenneth M
, et al.
JACS Au,2024,4(11):4249-4262.
DOI:
10.1021/jacsau.4c00577
More
Abstract: A Taxus baccatin III:3-amino-3-phenylpropanoyltransferase (BAPT, Accession: AY082804) in clade 6 of the BAHD family catalyzed a Mg2+-dependent transfer of isoserines from their corresponding CoA thioesters. An advanced taxane baccatin III on the paclitaxel biosynthetic pathway in Taxus plants was incubated BAPT and phenylisoserine CoA or isobutenylisoserinyl CoA with and without MgCl2. BAPT biocatalytically converted baccatin III to its 13-O-phenylisoserinyl and 3-(1',1'-dimethylvinyl)isoserinyl analogs, an activity that abrogated when Mg2+ ions were omitted. Baccatin III analogs that are precursors to new generation taxanes were also assayed with BAPT, the Mg2+ cofactor, and 3-(1',1'-dimethylvinyl)isoserinyl CoA to make paclitaxel derivatives at kcat/KM ranging between 27 and 234 s?1 M?1. Molecular dynamics simulations of the BAPT active site modeled on the crystal structure of a BAHD family member (PDB: 4G0B) suggest that Mg2+ causes BAPT to use an unconventional active site space compared to those of other BAHD catalysts, studied over the last 25 years, that use a conserved catalytic histidine residue that is glycine in BAPT. The simulated six-membered Mg2+?coordination complex includes an interaction that disrupts an intramolecular hydrogen bond between the C13-hydroxyl and the carbonyl oxygen of the C4-acetate of baccatin III. A simulation snapshot captured an active site conformation showing the liberated C13-hydroxyl of baccatin III poised for acylation by BAPT through a potential substrate-assisted mechanism.
Keywords:
new-generation taxanes ;
BAHD biocatalysis ;
molecular dynamics ;
Mg2+-dependence ;
paclitaxel
Purchased from AmBeed:
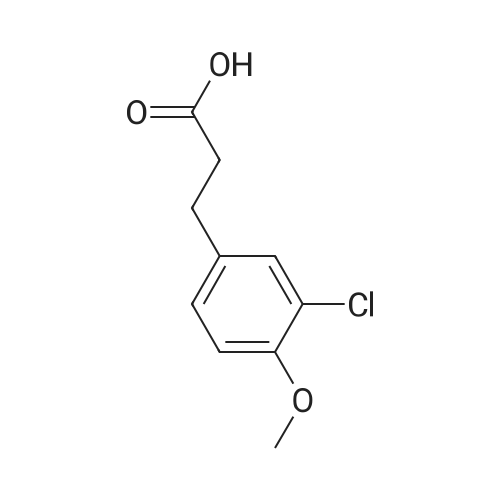
85-61-0

Biocatalytic and Regioselective Exchange of 2‐O‐Benzoyl for 2‐O‐(m‐Substituted) Benzoyl Groups to Make Precursors of Next‐Generation Paclitaxel Drugs
Aimen Al-Hilfi
;
Zhen Li
;
Kenneth M. Merz Jr.
, et al.
ChemCatChem,e202400186.
DOI:
10.1002/cctc.202400186
More
Abstract: A taxane 2-O-benzoyltransferase (mTBT, derived from Accession: AF297618) biocatalyzed the dearoylation and rearoylation of next-generation taxane precursors of drugs effective against multidrug-resistant cancer cells. Various taxanes bearing an acyl, hydroxyl, or oxo group at C13 were screened to assess their turnover by mTBT catalysis. The 13-oxotaxanes were the most productive, where 2-O-debenzoylation of 13-oxobaccatin III was turned over faster compared to 13-oxo-10-O-(n-propanoyl)-10-O-deacetylbaccatin III and 13-oxo-10-O-(cyclopropane carbonyl)-10-O-deacetylbaccatin III, yielding ~20?mg of each. mTBT catalysis was likely affected by an intramolecular hydrogen bond with the C13?hydroxyl. Oxidation to the 13-oxo recovered catalysis. The experimental data for the debenzoylation reaction was supported by Gaussian-accelerated molecular dynamics simulations that evaluated the conformational changes caused by different functional groups at C13 of the substrate. These findings also helped postulate where the 2-O-benzoylation reaction occurs on the paclitaxel pathway in nature. mTBT rearoylated the debenzoylated 13-oxobaccatin III acceptors fastest with a non-natural 3-fluorobenzoyl CoA among the other aroyl CoA thioesters evaluated, yielding ~10?mg of each with excellent regioselectivity at laboratory scale. Reducing the 13-oxo group to a hydroxyl yielded key modified baccatin III precursors (~10?mg at laboratory scale) of new-generation taxoids.
Purchased from AmBeed:
85-61-0


 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping