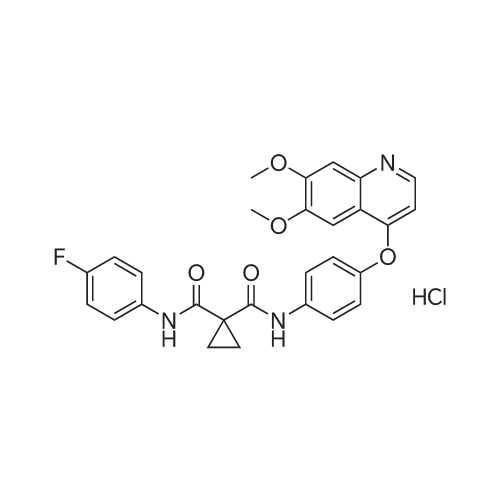
Alternatived Products of [ 849217-68-1 ]
Product Details of [ 849217-68-1 ]
| CAS No. : | 849217-68-1 |
MDL No. : | |
| Formula : |
C28H24FN3O5
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | ONIQOQHATWINJY-UHFFFAOYSA-N |
| M.W : |
501.51
|
Pubchem ID : | 25102847 |
| Synonyms : |
XL 184; BMS 97351
|
Chemical Name : | N-(4-((6,7-Dimethoxyquinolin-4-yl)oxy)phenyl)-N-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide |
Safety of [ 849217-68-1 ]
Application In Synthesis of [ 849217-68-1 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 849217-68-1 ]
- 1
-
 [ 849217-54-5 ]
[ 849217-54-5 ]

-
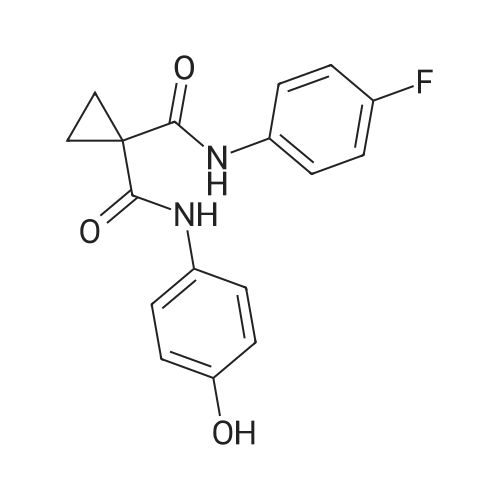 [ 849217-60-3 ]
[ 849217-60-3 ]

-
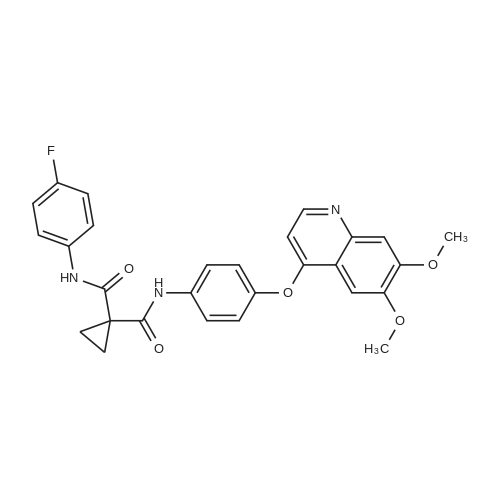 [ 849217-68-1 ]
[ 849217-68-1 ]
| Yield | Reaction Conditions | Operation in experiment |
| 44% |
With 2,6-dimethylpyridine; at 165℃; for 18h;Sealed tube; |
6,7-Dimethoxyquinolin-4-yl trifluoromethanesulfonate (5 g, 14.8 mmol) was added to a solution ofN- (4-fluorophenyl) -N '- (4-hydroxyphenyl) -cyclopropane-1,1-dicarboxamide (6.98 g, 22.2 mmol)2,6-lutidine (50 ml).The reaction mixture was stirred in a borning jar at 165 C for 18 hours.The reaction mixture was evaporated to dryness under reduced pressure and the remaining solid was dissolved in dichloromethane (250 ml)Wash twice with aqueous sodium hydroxide (1 N).The crude product was passed through a column of solid N- [4 - [(6,7-dimethoxy-Quinolyl) oxy] phenyl] -N '- (4-fluorophenyl) -1,1- cyclopropane dicarboxamide (3.2 g, yield: 44%),Captopinib |
| 44% |
With 2,6-dimethylpyridine; at 165℃; for 18h; |
To a solution of cyclopropane-l, L-DICARBOXYLIC acid (4- fluoro-phenyl) -amide (4-hydroxy-phenyl) -amide (6.98 g, 22.2 mmol) in anhydrous 2,6- lutidine (50 mL) was added trifluoromethanesulfonic acid 6,7-dimethoxy-quinolin-4-yl ester (5 g, 14.8 mmol). The reaction mixture was heated at 165C in a sealed pressure tube with stirring for 18 h. The reaction mixture was concentrated on high vacuum to completely remove lutidine. The resulting solid material was dissolved in DCM (250 mL), and washed several times with 1 N sodium hydroxide to remove the excess phenol. The crude mixture was loaded on a silica gel flash column and eluted with 75% EtOAc- hexanes, affording N- (4- { [6,7-bis (methyloxy) quinolin-4-yl] OXY} PHENYL)-N- (4- fluorophenyl) cyclopropane-l, L-DICARBOXAMIDE (3.2 g, 44%). |
- 2
-
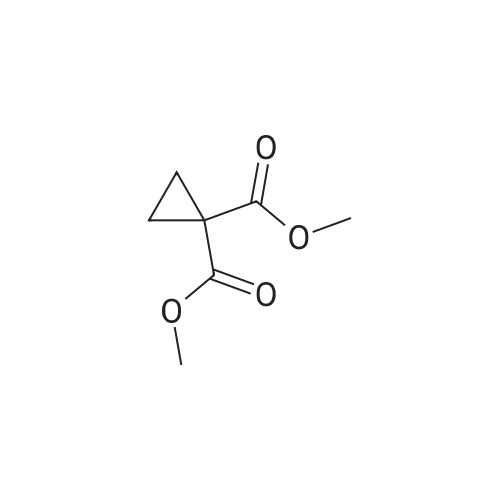 [ 6914-71-2 ]
[ 6914-71-2 ]

-
 [ 849217-68-1 ]
[ 849217-68-1 ]
- 3
-
 [ 849217-60-3 ]
[ 849217-60-3 ]

-
6,7-dimethoxy-2-carboxy-4-chloroquinoline
[ No CAS ]

-
 [ 849217-68-1 ]
[ 849217-68-1 ]
| Yield | Reaction Conditions | Operation in experiment |
| 65% |
With quinoline; copper; at 60 - 140℃; for 8h; |
500mL four-neck bottle,Add quinoline (200g),Copper powder (0.96g, 0.015mol)And a compound of formula IV (47.3 g, 0.15 mol),Warming up to 60 C,After the addition of 6,7-dimethoxy-2-carboxy-4-chloro-quinoline (formula VIII, 40.2 g, 0.15 mol),The temperature was further raised to 135-140 C for 8 hours.Add toluene (200mL) after coolingDilute the reaction solution,Transfer to a 1000mL four-neck bottle.Add 3% hydrochloric acid solution (36% hydrochloric acid 22.8g, 250.8g water),After stirring, the layers are extracted,The aqueous layer was collected and washed twice with toluene (100 mL*2).The aqueous layer was collected and decolorized and filtered with activated carbon. The filtrate was diluted with THF (125 mL), and the pH was adjusted to be more than 9 with a sodium carbonate solution to precipitate a solid, which was filtered. The filter cake was washed with a mixed solvent of THF (90 mL) and water (180 mL), and filtered. The filter cake was rinsed with a mixture of THF (20 mL) and water (40 mL) and filtered. The filter cake was dried overnight at 55 C to give cabozantini (Form I, pale yellow solid, 48.9 g, 65.0%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping