| 64% |
|
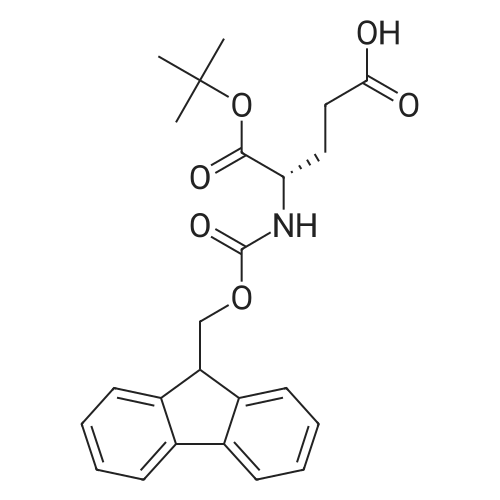
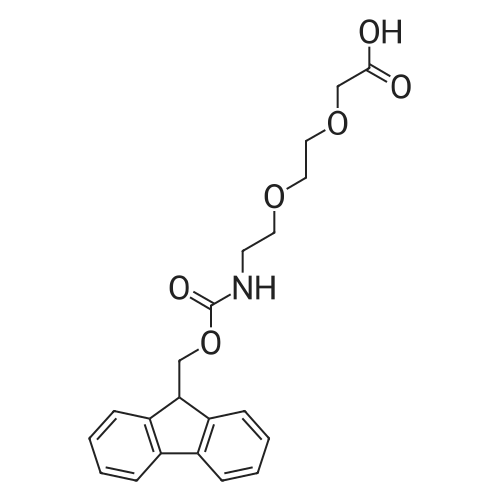
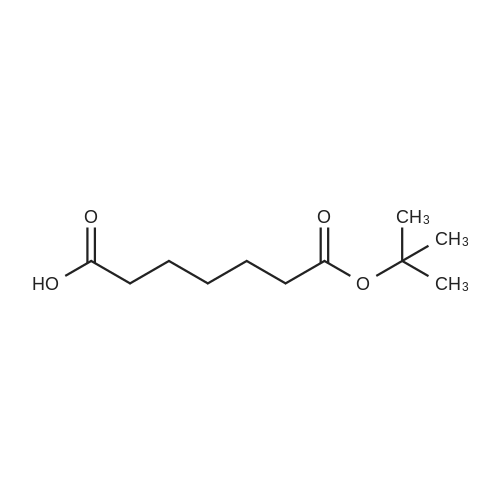
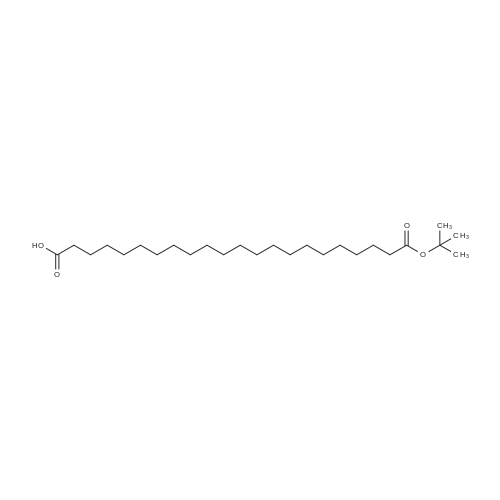
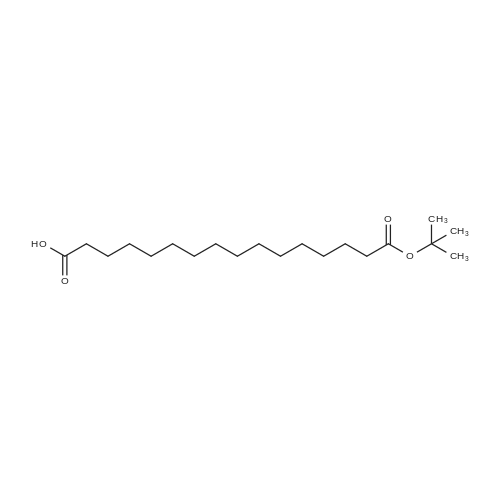
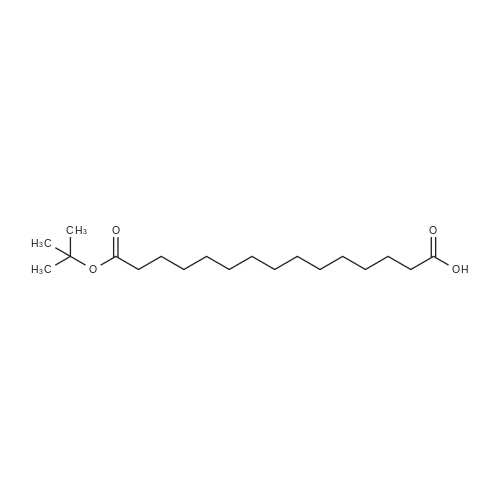
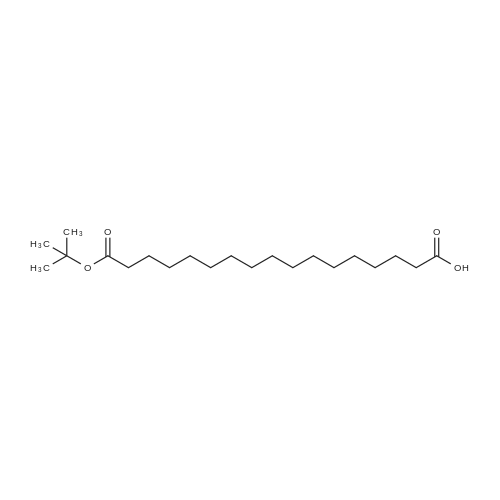
Synthetic protocol: Wang Fmoc-Lys(Mtt) resin 0.26 mmol/g (1, 11.7 g, 3.05 mmol) was left to swell in 5 dichloromethane (100 ml) for 45 minutes. Fmoc group was removed by treatment with 20% piperidine in N,N-dimethylformamide (1 x 5 min, 1 x 10 min, 1 x 30 min, 3 x 90 ml). Resin was washed with N,N-dimethylformamide (3 x 90 ml), 2-propanol (3 x 90 ml) and dichloromethane (3 x 90 ml). A solution of {2-[2-(9H-fluoren-9ylmethoxycarbonylamino)-ethoxy]-ethoxy}-acetic acid (Fmoc-OEG-OH, 2.35 g, 6.09 10 mmol), 0-( 6-chlorobenzotriazol-1-yi)-N, N,N', N' -tetramethyluronium tetrafluoroborate (TCTU, 2.17 g, 6.09 mmol) and N,N-diisopropylethylamine (2.12 ml, 12.2 mmol) in N,Ndimethylformamide (100 ml) was added to resin and the mixture was shaken for 1 hour. Resin was filtered and washed with N,N-dimethylformamide (3 x 90 ml), dichloromethane (3 x 90 ml) and N,N-dimethylformamide (3 x 90 ml). Fmoc group was 15 removed by treatment with 20% piperidine in N,N-dimethylformamide (1 x 5 min, 1 x 10 min, 1 x 30 min, 3 x 90 ml). Resin was washed with N,N-dimethylformamide (3 x 90 ml), 2-propanol (3 x 90 ml) and dichloromethane (3 x 90 ml). Solution of {2-[2-(9Hfluoren-9-ylmethoxycarbonylamino)-ethoxy]-ethoxy}-acetic acid (Fmoc-OEG-OH, 2.35 g, 6.09 mmol), 0-(6-chloro-benzotriazol-1-yi)-N,N,N',N'-tetramethyluronium 20 tetrafluoroborate (TCTU, 2.17 g, 6.09 mmol) and N,N-diisopropylethylamine (2.12 ml, 12.2 mmol) in N,N-dimethylformamide (100 ml) was added to resin and mixture was shaken for 1.5 hour. Resin was filtered and washed with N,N-dimethylformamide (3 x 90 ml), dichloromethane (3 x 90 ml) and N,N-dimethylformamide (3 x 90 ml) to obtain intermediate 1. Fmoc group was removed by treatment with 20% piperidine in N,N25 dimethylformamide (1 x 5 min, 1 x 10 min, 1 x 30 min, 3 x 90 ml). Resin was washed with N,N-dimethylformamide (3 x 90 ml), 2-propanol (3 x 90 ml) and dichloromethane (3 x 90 ml). Solution of (S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-pentanedioic acid 1-tert-butyl ester (Fmoc-LGiu-OtBu, 1.94 g, 4.57 mmol), 0-(6-chloro-benzotriazol-1-yi)N,N,N',N'-tetramethyluronium tetrafluoroborate (TCTU, 1.62 g, 4.57 mmol) and N,N30 diisopropylethylamine (1.43 ml, 8.23 mmol) in N,N-dimethylformamide (100 ml) was wo 2017/220706 PCT/EP2017/065342 72 added to resin and mixture was shaken for 1.5 hour. Resin was filtered and washed with N,N-dimethylformamide (3 x 90 ml), dichloromethane (3 x 90ml) and N,Ndimethylformamide (3 x 90 ml). Fmoc group was removed by treatment with 20% piperidine in N,N-dimethylformamide (1 x 5 min, 1 x 10 min, 1 x 30 min, 3 x 90 ml). 5 Resin was washed with N,N-dimethylformamide (3 x 90 ml), 2-propanol (3 x 90 ml) and dichloromethane (3 x 90 ml). Solution of 4-[(9H-fluoren-9ylmethoxycarbonylamino )methyl]cyclohexanecarboxylic acid (<strong>[188715-40-4]Fmoc-Trx-OH</strong>, 1. 73 g, 4. 57 mmol), 0-( 6-chloro-benzotriazol-1-yi)-N,N,N', N'-tetramethyluronium tetrafluoroborate (TCTU, 1.62 g, 4.57 mmol) and N,N-diisopropylethylamine (1.43 ml, 8.23 mmol) in N,N10 dimethylformamide (100 ml) was added to resin and mixture was shaken for 1 hour. Resin was filtered and washed with N,N-dimethylformamide (3 x 90 ml), dichloromethane (3 x 90ml) and N,N-dimethylformamide (3 x 90 ml) to obtain intermediate2. Fmoc group was removed by treatment with 20% piperidine in N,Ndimethylformamide (1 x 5 min, 1 x 10 min, 1 x 30 min, 3 x 50 ml). Resin was washed 15 with N,N-dimethylformamide (3 x 50 ml), 2-propanol (3 x 50 ml) and dichloromethane (3 x 30 ml). Solution of octadecanedioic acid mono-tert-butyl ester (C18(0tBu)-OH, 0.85 g, 2.28 mmol), 0-(6-chloro-benzotriazol-1-yi)-N,N,N',N'-tetramethyluronium tetrafluoroborate (TCTU, 0.81 g, 2.28 mmol) and N,N-diisopropylethylamine (0. 72 ml, 4.11 mmol) in N,N-dimethylformamide (50 ml) was added to resin and mixture was 20 shaken for 1.5 hour. Resin was filtered and washed with N,N-dimethylformamide (3 x 50 ml), dichloromethane (3 x 50 ml) and N,N-dimethylformamide (3 x 50 ml). Mtt group was removed by treatment with 80% 1,1,1,3,3,3-hexafluoro-2-propanol in dichloromethane (2 x 10 min, 2 x 30 min, 4 x 50 ml). Resin was washed with dichloromethane (6 x 50 ml). Solution of bromoacetic acid (4.24 g, 30.5 mmol) and 25 N,N '-diisopropylcarbodiimide (DIC, 4.01 ml, 25.9 mmol) in N,N-dimethylformamide (50 ml) was added to resin and mixture was shaken for 45 minutes. Resin was filtered and washed with N,N-dimethylformamide (5 x 50 ml) and dichloromethane (10 x 50 ml). The product was cleaved from resin by treatment with trifluoroacetic acid (50 ml) for 1 hour. Resin was filtered off and washed with trifluoroacetic acid (1 x 25 ml) and 30 dichloromethane (2 x 30 ml). Solutions were combined and solvents were evaporated to dryness giving the compound as thick brownish oil. Yield: 2.18 mg (64%). 1H NMR spectrum (300 MHz, Ac0D-d4, 80C, dH): 4.72-4.55 (m, 2 H); 4.16 (s, 2 H); 4.12 (s, 2 H); 3.80-3.62 (m, 12 H); 3.58-3.44 (m, 4 H); 3.32 (t, J=6.8 Hz, 2 H); 3.15 35 (d, J=6.8 Hz, 2 H); 2.51-2.07 (m, 8 H); 2.01-1.77 (m, 6 H); ... |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping